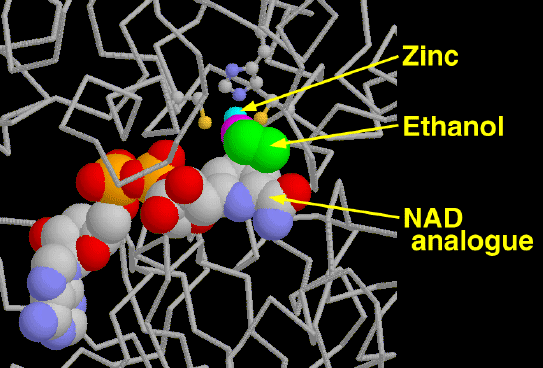
Alcohol dehydrogenase is our primary defense against alcohol, a toxic molecule that depresses the nervous system. High concentrations of alcohol dehydrogenase in the liver and stomach detoxify about approximately one ounce of liquor per hour. The alcohol is converted to acetaldehyde by the oxidation of NAD+ to NADH. Acetaldehyde is even more toxic to the body than ethanol. The body quickly convertes the acetaldehyde into acetate and other molecules that are easily utilized by the cell. Ethanol is not the only target of these enzymes, they also make important modifications to retinol, steroids, and fatty acids.