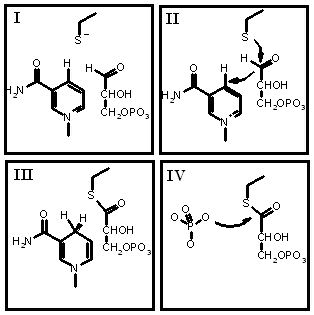
During glycolysis, gyceraldehyde-3-phosphate dehydrogenase converts glyceraldehyde-3-phosphate (G-3-P) to 1,3-bisphosphoglycerate (1,3-BGP) through oxidative phosphorylation. NAD+ is oxidized to NADH when a Pi is exchanged for a hydrogen on the carbonyl carbon of G-3-P thus converting G-3-P to 1,3-BGP.