Overview
Amylase belongs to the group of hydrolases known as glycosylases, which hydrolyze glycosyl compounds. Dietary carbohydrates enter the body as disaccharides, starches (amylose and amylopectin), and as glycogen. The first step of carbohydrate metabolism is the conversion of the larger polymers to a more simple, smaller form that can be easily transported to the tissues. The breakdown of polymeric sugars begins in the slightly acidic (pH of 6.8) saliva of the mouth, which contains lingual amylase that begins the digestion of carbohydrates. The action of lingual amylase is limited to the area of the mouth and the esophagus, and it is virtually inactivated by the acid of the stomach.
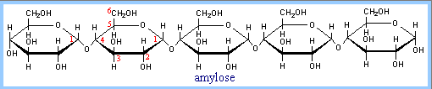
Once the food has arrived in the stomach, acid hydrolysis contributes to its degradation. From the stomach, the saliva, food, and other gastric secretions move to the small intestine where the main digestive enzyme a-amylase dwells. This enzyme is secreted by the pancreas and has the same activity as salivary amylase, producing disaccharides and trisaccharides by the hydrolysis of a-(1,4) glycosidic linkages (Figure 1) of starch components, glycogen, and various oligosaccharides. The net result is the almost complete conversion of carbohydrate to monosaccharides.
The consists of three caboxylic acids: Asp 174, Glu 200, and Asp 264. The active site uses two of the carboxylic acids in order to cleave the glycosidic bonds using a double displacement mechanism. The residue acts as a catalytic nucleophile in this mechanism; the acts as a general acid catalyst; the residue stabilizes the protonated state of the Glu 200 sidechain.