Alpha amanitin is a molecule made from the “death cap” mushroom and is a known potent inhibitor RNA polymerase. One single mushroom could very easily lead to a fast death in 10 days.

The mechanism of action is that alpha amanitin inhibits RNA polymerase at both the initiation and elongation states of transcription. The binding site for the inhibitor is the cleft between Rpb1 and Rpb2 in RNA polymerase. The cleft formed between these two subunits is connected to a helix bridge. The alpha amanitin toxin binds just beneath the bridge restricting the movement of the bridge by interactions between amanitin and residues 726, 767, 768, 769, and 822 of Rpb1 and by hydrogen bonding to residue 763 and 765 of Rbp2 (Bushnell, et al, 2002).
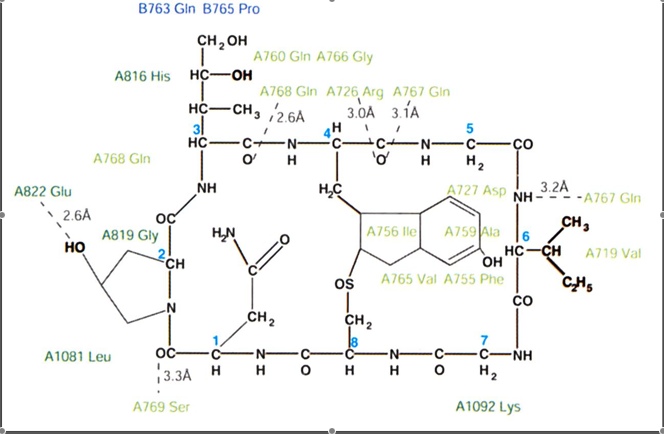
Figure 1: The chemical structure of alpha-amanitin showing where the toxin binds to RNA polymerase (Bushnell, et al., 2002).
The binding of a-amanitin toxin restricts the movement of the helix bridge, and has no effect on the flow of nucleotides to the active site. This restricted movement of the helix bridge causes the rate of translocation to slow down tremendously which results in strain place on the bridge helix. In order for RNA polymerase to move along the DNA strand the bridge helix must move, but when a-amanitin is bound to the bridge helix, it is unable to cause translocation (Bushnell, et al, 2002).