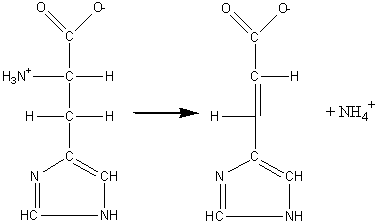
 | Lyases are a class of enzymes that cleave carbon-carbon, carbon-oxygen, phosphorous-oxygen, and carbon-nitrogen bonds by reactions than by hydrolysis or oxidation. This often forms a new double bond or ring structure. For example, the enzyme histidine ammonia-lyase catalyzes the reaction shown to the left which results in the formation of a double bond.
|
Another aspect of lyases that makes them different from most other enzymes is that they often only require one substrate for the forward reaction and two substrates for the reverse reaction.
The enzymes the make up the lyase class have a wide variety of functions. They can be involved in metabolic pathways, anabolic pathways, signal transduction, and even DNA repair. |
|
| To further understand how lyases function and their importance, three specific enzymes will be looked at in detail. They are tryptophan synthase, pectate lyase, and apurinic/apyrimidinic endonuclease. Tryptophan synthase is involved in the synthesis of tryptophan, an amino acid. Pectate lyase is released by cells as a weapon in order to break the cell walls of neighboring plant cells by cleaving the de-esterfied pectin that is present in the cell wall. Apurinic/apyrimidinic endonuclease is an essential enzyme in DNA base excision repair and works by cutting the DNA backbone. |
| Select a Lyase |