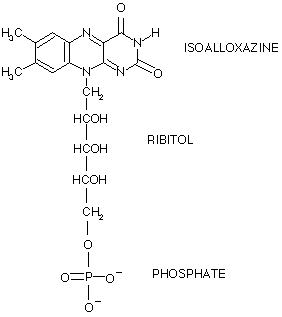
Flavin mononucleotide (FMN) is a cofactor that helps catalyze a wide range of reactions. It is also known as riboflavin 5'-phosphate. The structure of FMN is shown below.

The structure of FMN consists of three main parts, the isoalloxazine ring, the ribitol group, and a phosphate. The isoalloxazine ring is the core part of FMN. This part of FMN is capable of forming many hydrogen bonds to the enzymes that it is associated with. The ribitlyl group makes up the center portion of the cofactor, while the phosphate group is bonded to the end of the ribitol.
FMN can exist in any of three different redox states. When FMN is fully oxidized, it is yellow in color and absorbs light at 450nm. It can be converted to a semiquinone by a one-electron transfer. At a physiological pH, the semiquinone is a neutral radical that is blue in color and absorbs light at 570nm. It has a pka of around 8.4. At higher pH values it loses a proton and becomes a radical anion, which is red in color and absorbs light at 490nm. When FMN loses a second electron, it is converted into a completely reduced dihydroflavin that is colorless.
Since FMN is able to convert into these three different redox states, it can participate in one-electron transfer and two-electron transfer reactions. Because it is able to do this, it is able to catalyze a wide array of reactions, and also work with many other electron donors and acceptors. Some of these include two electron donor/acceptors like NAD+ and NADPH+, one or two-electron carriers like quinones, and one-electron donor/acceptors like cytochrome proteins.