In Part I of this Elaboration we developed an operational definition for acids and bases, which allows us to go into a lab and determine whether a substance is an acid or a base. In Part II of this Elaboration we developed the Arrhenious definition for acid and bases, which describes in chemical terms how acids and bases work to increase or decrease the pH of a solution. Here we will discuss that the Arrhenius definition does not account for all the substances, which according to operational definition, are acids an bases. Aqueous ammonia (NH3) is and example. When ammonia, which like hydrogen chloride (HCl) is a gas, is dissolved in water, it causes the the pH to rise. That make aqueous NH3 a base. This can be demonstrated with the Virtual Lab Simulator. To prove this to yourself, open the simulator and use it to make up a 0.01 M solution of aqueous NH3, and then measure its pH. You should obtain what is shown in Figure 1.
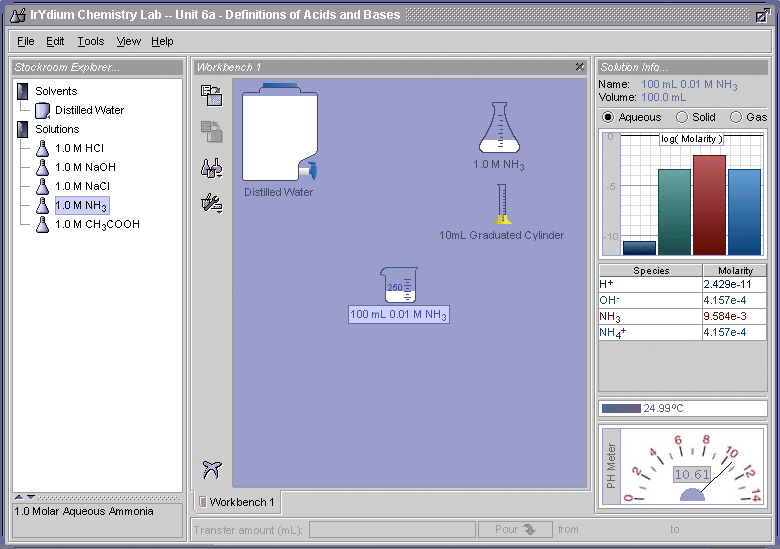 |
As the Virual Lab Simulator demonstrates, at pH 10.61, a 0.01 M aqueous solution of NH3 is basic. This means that the NH3 is somehow causing the OH- concentration to increase, but in trying to apply the Arrhenius definition, it is unclear how it does this.
![]()
The ammonia contains no hydroxyl ions to dissociate. The solution to this mystery is that after dissolving the water,
![]() ,
,
the aqueous ammonia then reacts with water. It does so by pulling a hydrogen ion off of a water molecule and leaving behind a hydroxyl ion.
![]()
The source of the hydroxyl ions turns out to be the water.
The Brønsted-Lowry Definition of Acids and Bases
In 1923, the Danish chemist Johannes Brønsted, and the English chemist Thomas Lowry, independently proposed a new definition for acids and bases that accounts for the behavior of ammonia.
  |
According to the Brønsted-Lowry definition, a base is a substance that accepts a hydrogen ion, or proton, from a Brønsted-Lowy acid, and an acid is a substance that donates a hydrogen ion, or proton, to a Brønsted-Lowry base. In this definition, a proton is considered to be equivalent to a hydrogen ion (H+).
This definition has a certain symmetry to it: the H2O in the equation shown above is acting as the acid because it is donating a proton to the NH3, while the NH3 is the base because it is accepting the proton from the H2O. Acid/Base reactions generally reach an equlilibrium very quickly. As a described in Section 9.3 of Raymond, at equlibrium the reaction is occuring at the same rate in both directions. To reflect this, we can use a doubled-headed arrow in the reaction equation:
![]()
If you consider this reaction in the reverse direction, you will see that the ammonium ion (NH4+) is donating a proton to the hydroxyl ion (OH-). Brønsted-Lowry acid/base reaction equations, therefore, always have an acid and a base on the left-hand side of the equation, plus an acid and a base on the right-hand side of the equation:
![]()
In this equation, the NH3 and NH4+ are called conjugates; NH4+ is the conjugate acid for the NH3. The H2O and OH- are likewise conjugates; OH- is the conjugate base for the H2O. Conjugates differ from one another by the presence or absence of a proton, H+.
We can now add the Brønsted-Lowry to our list of definitions:
| Acid | Base | |
| Operational Definition |
When added to water pH < 7 |
When added to water pH > 7 |
| Arrhenius Definition |
When added to water dissociates to release a hydrogen ion (H+) |
When added to water dissociates to release a hydroxyl ion (OH-) |
| Brønsted-Lowry Definition |
Donates a proton (H+) to a base. |
Accepts a proton (H+) from an base. |
Arrhenius acids can also be represented as a Brønsted-Lowry acid, for example, when hydrogen chloride (HCl) is dissolved in water, the hydrochloric acid, HCl (aq), donates a proton to water to produce a hydronium ion, H3O+.
![]()
Instead of producing a free hydrogen ion, when Brønsted-Lowry acids are dissolved in water they produce a hydrodium ion, H3O+. This is actually a more realistic way to represent an acid since protons do not exist free in solution, but attach themselves to a water molecule to produce hydronium ion, H3O+. When using the Brønsted-Lowry definition of acids and bases, the pH is determined from the the hydrodium ion concentration instead of the hydrogen ion concentration:
![]()
Also note that water is participating as an acid when it reacts with ammonia, NH3, and as a base when it reacts with hydrochloric acid. Compounds, such as water, that can act as either an acid or a base are called amphoteric.
In Part II of this Elaboration, we introducted the dissociation equation for water, which produced hydrogen ions and hydroxyl ion. The Brønsted-Lowry counterpart for this equation is
![]()
And the equilibrium constant expresssion for this reaction using the Brønsted-Lowry formalism is
![]()