In Chapter 2 of Horton et al. we saw that for reactions in which water is a reactant, the water molecules split into H+ and OH- ions. For this reason these reactions are hydrolysis ("water-split") reactions. When a water molecule splits, it produces a hydrogen ion (H+) and a hydroxyl ion (OH-). It turns out, that in pure water, there is also a small number of water molecules that split to form hydrogen and hydroxyl ions.
![]()
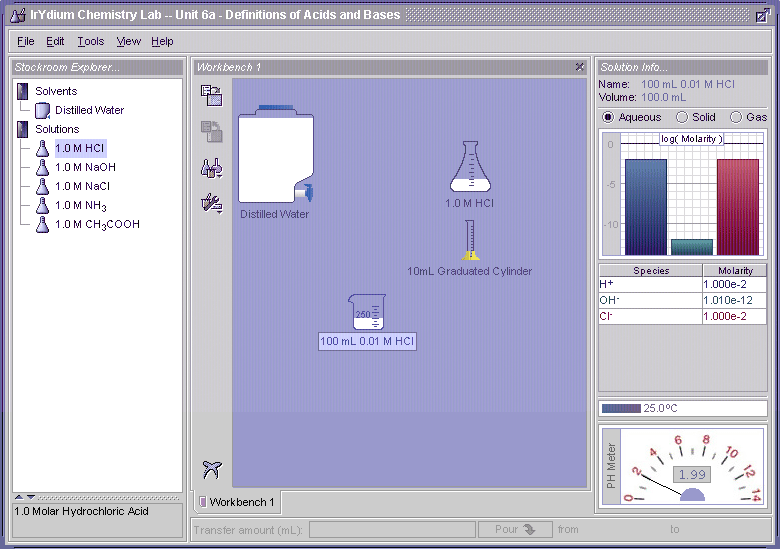
The concentrations of hydrogen ions and hydroxyls ions in pure water is quite low, at around 0.0000001 moles/liter = 1 x 10-7 moles/liter, which is much less than the molar concentration of pure water, which is 55.5 moles/liter. This means that only about one in every half-billion water molecules spit to form a hydrogen ion and a hydroxyl ion. Even though these numbers are very small, these species are still very reactive and their concentrations are very important in biological systems. For example, if the hydrogen ion concentration in blood, as expressed by pH, drops from 7.4 to 6.8, coma and death can result.
The pH is an alternative may of expressing hydrogen ion concentrations in solutions. The pH of a solution is defined as:
![]()
where [H+] represents the concentration of hydrogen ions in moles/liter. The pH values are often used instead of molar concentrations because they compress a rather wide range of concentration values, which for H+ can vary from 1 mole/liter to 0.000000000000001 mole/liter, to a pH value range that varies from 0 to 15. When this is applied to the hydrogen ion concentration in pure water:
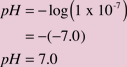
The Operational Definition of Acids and Bases
By "operational" we mean you do some kind of operation to determine if something is an acid is an acid or a base. For example, you could after dissolving a substance in water, then taste it. Typically acids will taste sour, while bases taste bitter. This is a rather subjective way of distintinguishing acids from bases, and is also risky from a health stand point. Another way to test for acids and bases is to use chemicals called acid-base indicators. These chemicals turn different colors in the presence of acids or bases. For example, the indicator litmus turns pink when exposed to acid and blue when exposed to base. And still another way is to use an instrument, called a pH meter, which measures the pH of a solution. When an acid is added to pure water, it has been found that the pH of the solution drops below 7.0. On the other hand, if a base is added to pure water, the pH of the solution rises above 7.0. These operational definitions of acids and bases are summarized in the table below:
| Acid | Base | |
| Operational Definition |
When added to water pH < 7 |
When added to water pH > 7 |
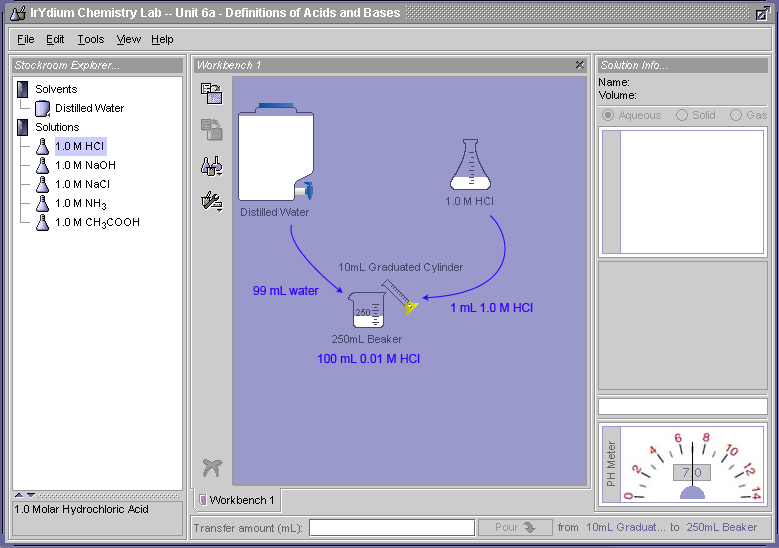
Let's strap on our safety goggles and go into the lab and try this out. Below is a virtual lab simulator which we will use to investigate the chemical properties of acids and bases. You can either follow along by using the lab shown in Figure 1, or open the lab up in a separate window by clicking here.