In Part I of this Elaboration we developed an operational definition for acids and bases, which allows us to go into a lab and determine whether a substance is an acid or a base: you put the substance in a beaker along with some water and measure the pH. If the pH is less than 7, the substance is an acid, if the pH is greater than 7 it is a base. In Part I we also introduced the concept of pH as a measure of the hydrogen ion concentration:
![]()
where [H+] represents the hydrogen ion concentration in units of moles/liter. For pure water at room temperature the pH is 7.0. This is because the hydrogen ion concentration in pure water at room temperature is 1.0 x 10-7 mole/liter and -log(1.0 x 10-7) = 7.0. The source of the hydrogen ions is the water itself:
![]()
About 1 in every half-billion molecules in pure water will split to produce a H+ ions. Note also, that this splitting of water molecules also produces a hydroxyl ion, therefore, in pure water, the hydroxyl ion concentration is also 1.0 x 10-7 mole/liter.
In Sections 9.3 and 9.5 in Raymond we learn that this splitting of water into hydrogen ions and hydroxyl ions is an equilibrium that can be characterized by an equilibrium constant, Keq. In the case of water, it is equal to the product of the hydrogen ion concentration times the hydroxyl ion concentration.
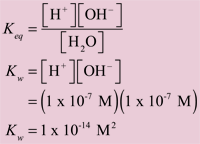
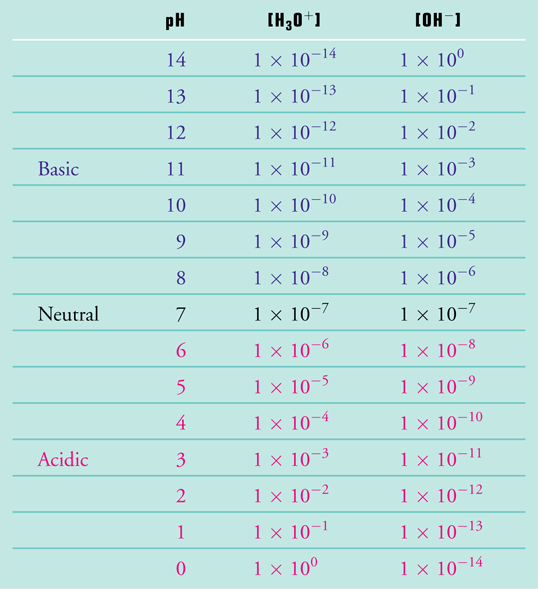
Because the water is present at such a high concentration relative to the hydrogen and hydroxyl ions (55.5 M for H2O versus 0.0000001 M for H+ and OH-), the 1 in half-billion water molecules that do decide to split have essentially no effect on the water's concentration. When the concentration of one of the components does not change in an equilibrium constant expression, it is dropped from the expression. In this case we also change the subscript of the equilibrium constant from eq to w. The equilibrium expression for water tells us that as the pH drops when an acid is added to water, the hydrogen ion concentration increases while the hydroxyl ion concentration decreases concomitantly so that the product [H+][OH–] remains equal to 1 x 10-14 M2. By this same reasoning, it also means that as the pH increases when a base is added to water, the hydrogen ion concentration decreases while the hydroxyl ion concentration increases to keep the product [H+][OH–] equal to 1 x 10-14 M2. The relationship between in [H+] and [OH–] is illustrated in Figure 1.
 |
The Arrhenius Definition of Acids and Bases
Our next definition of acids and bases is one that attempts to explain in chemical terms why a substance is an acid or base. This definition is an extension of a theory on electrolyte solutions that was put forward by the Swedish chemist, Svante Arrhenius, in his 1884 doctoral thesis (Figure 2).
 |
Interesting, Arrhenius was given the lowest possible passing grade for his thesis, however, the work later earned him the 1903 Nobel Prize in Chemistry! According to the Arrhenius theory, an acid is a substance that dissociates to release a hydrogen ion when dissolved in water. This causes the hydrogen ion concentration to increase and the pH to drop. Also, a base is substance that dissociates to release a hydroxyl ion when dissolved in water. This causes the hydroxyl ion concentration to increase, and according Figure 1, causes the hydrogen ion concentration to decrease. This is why the pH increases when a base is added to water. We can now add the Arrhenius definition to our list of definitions:
| Acid | Base | |
| Operational Definition |
When added to water pH < 7 |
When added to water pH > 7 |
| Arrhenius Definition |
When added to water dissociates to release a hydrogen ion (H+) |
When added to water dissociates to release a hydroxyl ion (OH-) |
In Part I of this Elaboration we used the Virtual Lab Simulator to measure the pH of 0.01 M solution of hydrochloric acid and found that the HCl caused the pH to drop below 7, making it an acid. According to the Arrhenius definition, it does this because when hydrogen chloride, which is a gas, HCl (g), is dissolved in water it dissociates to produce hydrogen ions and a chloride ions in solution.
![]()
Hydrochloric acid is a strong acid, which means that in solution it completely dissociates into hydrogen ions and chloride ions. Because the HCl solution that was made up in Part I had a concentration 0.01 M (1.0 x 10-2 M), it should have a hydrogen ion concentration of 1.0 x 10-2 M. We see from Figure 1, that this should produce a pH of 2.0. This is indeed the result we obtained with the Virtual Lab Simulator (see Figure 5, Part I of Elaboration - Definitions of Acids and Bases).
In the "Try This" to Part I of this Elaboration, you used the Virtual Lab Simulator to measure the pH of a 0.01 M NaOH. We saw That the NaOH caused the pH to rise above 7, making it a base. According to the Arrhenius definition, NaOH is a base because when dissolved in water, the NaOH dissociates to produce hydroxyl ions.
![]()
Sodium hydroxide is a strong base, which means that in solution it completely dissociates into the sodium ions and hydroxyl ions. Because the NaOH solution that was made up in Part I had a concentration 0.01 M (1.0 x 10-2 M), it should have a hydroxyl ion concentration of 1.0 x 10-2 M. We see from Figure 1, that this should produce a pH of 12.0. This is indeed the result we obtained with the Virtual Lab Simulator (see answer to the Try This", Part I of Elaboration - Definitions of Acids and Bases).
Many substances that dissociate to produce ions in solution are neither acids or bases. This is because when they dissociate they do not produce any hydrogen or hydroxyl ions. Such ionic substances are called neutral salts. Sodium chloride is a good example of a neutral salt. When dissolved in water, NaCl completely dissociates to produce sodium ions and chloride ions.
![]()
If you worked the "Try This" in Part I of this Elaboration, you used the Virtual Lab Simulator to measure the pH of a 0.01 M solution of NaCl. You should have observed that this had no effect on the pH (see answer to the "Try This", Part I of Elaboration - Definitions of Acids and Bases).