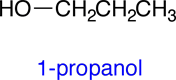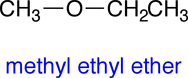Structural formulas are used to describe the 3-dimensional structures of molecules and polyatomic ions. Unlike molecular formulas, which only list the numbers of each type of atom found in a molecule, structural formulas show how the atoms are connected together in the molecule. There are several different conventions for drawing structural formulas, some of which are less explicit than others and require some knowledge of chemistry to infer the correct 3-dimensional structure. In this Elaboration we will start with the 3-dimensional structure of a molecule and then review the different structural formulas that can be used to communicate this 3-dimensional structures.
Below is a figure showing the 3-dimensional structure for a 2-propanol molecule. Later you will learn how to name organic molecules, such as 2-propanol; you are probably more familiar with this molecule by its more common name, rubbing alcohol.
The most explicit type of structural formula is one that we have already used, the electron dot structural formula; we used electron dot structures earlier to demonstrate how each atom in a molecule has fulfilled the octet rule (See Elaboration - The Octet Rule). Shown below is the electron dot structural formula for 2-propanol:
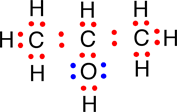 |
This structural formula can be made less cluttered by using lines to represent the the pairs of electrons that are participating in the covalent bonds. This produces the line-bond structural formula in which each line represents a pair of bonding electrons. Shown below is the line-bond structural formula for 2-propanol:
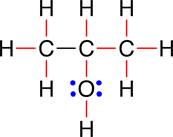 |
Showing the non-bonding electrons as dots in the line-bond structural formula is optional and can be omitted:
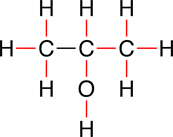 |
Here, the non-bonding electrons on the oxygen are inferred. The octet rule tells us that there should be 8 valence electron around the oxygen. The two covalent bonds, between the oxygen and the carbon, and between the oxygen and the hydrogen, account for 4 electrons, therefore, the oxygen has two additional pairs of non-bonding electrons.
The line-bond structural formula can be simplified further to produce a condensed structural formula. Since the hydrogen atoms can only form a single covalent bond to another atom, there is no need to explicitly show how the hydrogens bond to other atoms. By convention, the chemical symbol for hydrogen is placed next to the chemical symbol for the atom it is bonded to and a subscript is used to indicated the number of hydrogens that are bonded to a particular atom. Shown below is the condensed structural formula for 2-propanol:
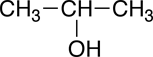 |
The condensed structural formula is often condensed even further by omitting the line-bonds between the carbon atoms:
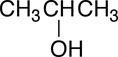 |
Because the hydrogens can only form one covalent bond, it is understood in the condensed structural formula shown above that the three carbons are bonded directly to one another and not through a hydrogen atom, i.e., a situation like this, C-H-C, is forbidden.
The final structural formula that we will introduce requires the greatest amount of chemical knowledge to correctly interpret, but on the upside, its simplicity makes it the most useful one for communicating structural information about molecules over the Internet. The condensed structural formula can be simplified even further to produce what is called a skeletal structural formula. Most of the molecules that we encounter in biochemistry are organic molecule, which contain more carbons and hydrogens than any other type of atom. In a skeletal structural formula, chemical symbols are only used to label the non-carbon atoms; if an atom is not labeled it is assumed to be a carbon atom. Also, the hydrogens that are bonded to carbons are not even shown. Since every carbon in a molecule should have 4 bonds connected to it, the number of hydrogen that are bonded to a carbon is assumed to be that number required to give each carbon a total of four bonds. Shown below is the skeletal structural formula for 2-propanol:
We will be using all these different forms of structural formulas this semester. The skeletal structural formula will be most useful because there is a web-based tool that we can use to draw skeletal structural formulas of molecules from a web page.