The molecular formula for 2-propanol is C3H8O. Your textbook mentions that there are two other molelcules that share this same molecular formula, but which have different structures. Later we will learn that molecules that have different structures and share the same the molecular formula are called isomers. Shown below are the condensed structural formulas for the other two isomers of 2-propanol: 1-propanol and methyl ethyl ether:
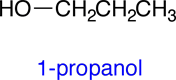 |
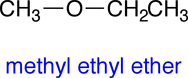 |
Draw the following structural formulas for these two molecules:
- Electron dot structural formula
- Line-bond structural formula
- Skeletal structural formula
a. Electron dot structural formula
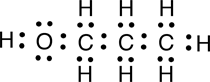 |
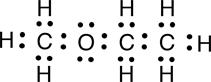 |
b. Line-bond structural formula
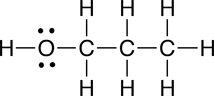 |
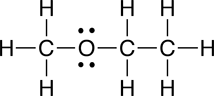 |
c. Skeletal structural formula