In Section 3.2 the concept of valence electrons was introduced. These are the electrons found in the highest occupied energy level or shell for an atom of an element. For our discussions we will focus on the elements in the first two columns on the left-hand side of the periodic table and the last six columns on the right-hand side of the table. Together, these elements are referred to as the main group or representative elements. Figure 3.6 in Raymond uses Lewis electron dot structures to show the number of valence electrons in some of the representative elements. Note that the number of valence electrons that each element has is equal to its group number, e.g., elements in Group IA have one valence electron (one dot), elements in Group IIA have two valence electrons (2 dots), etc.
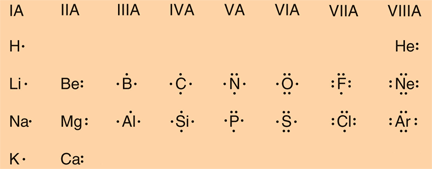 |
It turns out that there is something special in nature about having eight electrons in the valence shell. This happens be the most stable situation for an atom. The elements that have this number in their pure neutral forms are the elements in Group VIIIA. These elements, as a group, are are called the inert gases. All of the inert gases have 8 valence electrons, except helium, which has 2. This is because the first energy level can only hold 2 electrons, whereas the other energy levels can hold 8 or more electrons. (Each energy level can contain up to 2n electrons, where n is the energy level; refer to Section 3.2 and Table 3.4 in Raymond). Even if an energy level can hold more than 8 electrons, the most stable number, is 8. The inert gases are called inert because they are very unreactive with themselves and other elements. This reflects their high stability. All of the other elements on the periodic table would like to be like an inert gas in terms of the number of electrons they have in their valence shell. Chemistry can be thought of as basically a consequence of all of the elements on the periodic table trying to achieve the same number of valence electrons as one of the inert gases. They do this by reacting with one another to gain, lose or share electrons. so that each atom ends up with 8 electrons in their valence shell. This is a statement of what is called the octet rule.
The periodic table reflects this situation. Originally the elements were arranged on the periodic table, from left to right, according to their atomic number, and in columns according to their chemical and physical properties. For example the far right-hand column contains gases which are all chemically inert. Figure 3.8 in Raymond shows the representative elements:
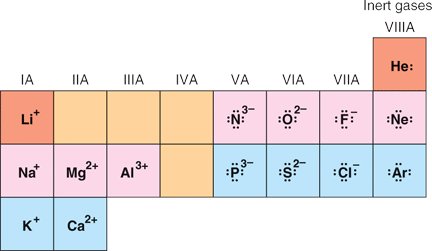 |
The periodic table also provides clues about an element's electronic structure. When focusing on the representative elements, the rows tell you which energy level contains the valence electrons: row 1 is the n=1 level, row two is the n=2 level, etc. The columns indicate, for the neutral form of the element, how many valance electrons are in the valance shell: the first column (Group IA) contains 1 valence electron, the second column (Group IIA) contains 2 valence electrons, etc., and the last column (Group VIIIA) contains 8 valence electrons.
The octet rule can be used to predict the charge on the monoatomic ions that form from the representative elements. This is illustrated in Figure 3.8. One of the ways that atoms obtain 8 electrons in their valence shell is to lose or gain electrons. The elements on the left-hand side of the periodic table tend to lose electrons, these are the metals, while the elements on the right-hand side of the periodic table tend to gain electrons, these are the non-metals. When metals and non-metals combine, the metals give their valence electrons to the non-metals, so that each in the end has 8 electrons in their outer energy level. In the process, the metals form positive ions (cations), while the non-metals form negative ions (anions). Figure 3.8 shows the Lewis electron dot structures for the common ions formed by the representative elements. The non-metals, located on the right-side of the periodic table, have each gained a number electrons equal to their charge, and as shown by the Lewis dot structures each has 8 electrons in their valence shell. The metals, located on the left-hand side of the periodic table, have each lost a number of electrons equal to their charge (now positive), and as shown by the Lewis dot structures, each has 0 electrons left in their valence shell! The consequence of this is that the next lower energy level, which is now the highest occupied energy level, will have 8 electrons in it (Look at Table 3.4 in Raymond).
Atoms and ions that share the same number electrons are said to be isoelectronic. Li+ is isoelectronic with He (2 electrons), and this is shown by the red boxes in Figure 3.8; N3-, O2-, F-, Na+, Mg2+ and Al3+ are all isoelectronic with Ne (10 electrons) and this is shown by the pink boxes in Figure 3.8; and P3-, S2-, Cl-, K+ and Ca2+ are isoelectronic with Ar (18 electrons) and this is shown by the blue boxes in Figure 3.8. Application of the octet rule predicts that these are the ions that should form from the representative elements.
Carbon (C) and silicon (Si) are in Group IVA. (See Figure 3.2 of Raymond, which is also shown above.) Elements in this group have 4 valence electrons. These elements could either gain or lose 4 electrons to become a monoatomic ion that is isoelectronic with an inert gas. However, because of the high charge that would result, either C4+ or C4- for carbon and Si4+ or Si4- for silicon, this is unlikely to happen. This is why they are not shown in the Group IVA column of Figure 3.8, shown above. We will see that carbon uses another strategy for adhering to the octet rule.
The octet rule does not work for predicting the charges on transition metals ions. Transition metals are located on the periodic table in the ten columns between columns IIA and IIIA for the representative elements, and the groups are labeled IB to VIIIB. This is shown in Figure 2.6 of Raymond:
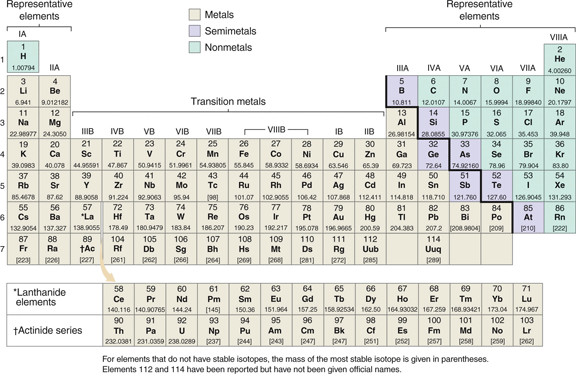 |
The transition metals typically produce ions with 1+, 2+, 3+ and sometime 4+ charges, and unlike the representative elements many transition metals can have more than one charge state. An important example in biochemistry is iron (Fe), which is typically found in either a Fe2+ or Fe3+ state. Certain proteins called electron transport proteins transport electrons by having bound iron ions cycle between a Fe2+ and Fe3+ states.