|
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Hartselab Research-Past and Present
Amphotericin
B is an antifungal and potentially anti-AIDS drug used to
treat
nasty fungal infections frequently seen in AIDS, chemotherapy and
transplant
patients.
Health professionals ruefully refer to this highly toxic yet very
effective
drug as "amphoterrible." In the past, the usual model for the
mechanism
of action of Amphotericin has invoked a "barrel stave" organization of
Amphotericin + sterol molecules forming a fungus-killing membrane pore.
Our research (1,2,3)
has proven conclusively that specific cation selective pores do not
need sterol to form. To paraphrase Huxley, this was an example of the
"Tragedy
of Science: a beautiful Hypothesis slain by an an ugly little
Fact."
Some researchers have protested our iconoclasm, but the results have
been
confirmed again
and again.
In addition we have shown that pores formed in the presence of the
fungal
sterol ergosterol are structurally and functionally different (4).
More recently, we have explored new, simple (and practical!) ways of
reducing
the toxicity of this drug (5,6).
This heat-treatment method of reducing toxicity has recently been
proposed
as a practical, inexpensive way to treat serious fungal diseases in
Thrird
World contries (7).
It's nice when something we do here in Eau Claire might actually have
global
benefits! For more on fungal infections and treatments (including cost
analyses), see my favorite web site, Dr.
Fungus!
The longest ongoing project
in our lab is to tease apart which specific properties
of Amphotericin B pharmaceutical preparations make them less toxic
(and
more effective) in hopes of finding a simpler way to increase the
drug's
therapeutic index. We are specifically interested in how
Liposomal and other supramolecular formulations of Amphotericin B
alter
toxicity and channel formation properties of this drug.  We use stopped-flow fluorescence, absorbance and CD methods to probe
the
structure and activity of Amphotericin preparations. In addition we are
beginning studies aimed at characterizing the biological response
of
monocytes to stimulation by different formulae. We propose that there
are
essentially three factors which influence the toxicity and efficacy of
AmB preparations: 1) direct membrane toxicity via ion channel
formation,
2) differences in distribution and delivery to tissues due to
differences
in serum lipoprotein/protein binding, and 3) initiation of an
inflammatory
cytokine response.
We use stopped-flow fluorescence, absorbance and CD methods to probe
the
structure and activity of Amphotericin preparations. In addition we are
beginning studies aimed at characterizing the biological response
of
monocytes to stimulation by different formulae. We propose that there
are
essentially three factors which influence the toxicity and efficacy of
AmB preparations: 1) direct membrane toxicity via ion channel
formation,
2) differences in distribution and delivery to tissues due to
differences
in serum lipoprotein/protein binding, and 3) initiation of an
inflammatory
cytokine response.
Another interetsing feature of Amphotericin B (AmB) is that it
is one
of the few agents shown to slow the course of prion diseases
(like chronic
wasting disease and mad cow disease) in animals. Prions and amyloid
diseases
like Alzheimer's have many features in common.
Chiefly, both diseases involve protein misfolding events which can induce other proteins to misfold into
largely
beta-sheet fibrillar or oligomeric isoforms (see EM photo of fibrils).
These structures seem to be the key to the disease pathogenesis by
various
proposed mechanisms. Congo Red is a dye that has been reported to
directly
inhibit amyloidogenesis in both prion and Alzheimer peptide model
systems
by specific binding. This binding affinity has long been used as a
spectroscopic
marker for amyloid protein. We propose it is possible that AmB may act
similarly to physically prevent fibril formation in prion disease. To
assess
whether AmB is capable of binding specifically to amyloid fibrils as
does
Congo Red, we have used the insulin fibril and amyloid precursor
protein
peptide (from Alzheimers) amyloid model system. In addition, AmB
interacts specifically with Congo Red, a known fibril-binding agent. In
kinetic fibril formation studies, AmB was able to significantly
kinetically
delay the formation of Abeta 25-35 fibrils and their final extent at pH
7.4 but not insulin fibrils at pH 2 at near therapeutic AmB levels.The
polyol region of AmB suggests that it could be an effective hydrogen
bond
donor/acceptor and may be able to terminate and/or stabilize the
beta-sheet
structure of a growing amyloid fibril. We are interested in physically
explaining AmB's unique actvitiy in the hopes that it could lead to
therapeutic
strategies for both prion and amyloid diseases(8).
misfolding events which can induce other proteins to misfold into
largely
beta-sheet fibrillar or oligomeric isoforms (see EM photo of fibrils).
These structures seem to be the key to the disease pathogenesis by
various
proposed mechanisms. Congo Red is a dye that has been reported to
directly
inhibit amyloidogenesis in both prion and Alzheimer peptide model
systems
by specific binding. This binding affinity has long been used as a
spectroscopic
marker for amyloid protein. We propose it is possible that AmB may act
similarly to physically prevent fibril formation in prion disease. To
assess
whether AmB is capable of binding specifically to amyloid fibrils as
does
Congo Red, we have used the insulin fibril and amyloid precursor
protein
peptide (from Alzheimers) amyloid model system. In addition, AmB
interacts specifically with Congo Red, a known fibril-binding agent. In
kinetic fibril formation studies, AmB was able to significantly
kinetically
delay the formation of Abeta 25-35 fibrils and their final extent at pH
7.4 but not insulin fibrils at pH 2 at near therapeutic AmB levels.The
polyol region of AmB suggests that it could be an effective hydrogen
bond
donor/acceptor and may be able to terminate and/or stabilize the
beta-sheet
structure of a growing amyloid fibril. We are interested in physically
explaining AmB's unique actvitiy in the hopes that it could lead to
therapeutic
strategies for both prion and amyloid diseases(8).
More recently, Dr.Turtinen and I have collaborated on the molecular
mechanism of AmB's ability to induce a cytokine response and how
drug delivery
systems can modify this (9).
We have found that there are essentially two categories of AmB drug
delivery
preparations; those that stimulate TNF-a and
IL-6 in monocytes and those that do not. We use new chemiluminescent
antibody-array
detection (see illustration).
Why would anyone care? Well, TNF and IL-6 both stimulate
HIV replication. So if you are prescribing antifungal medication to an
AIDS patient with serious fungal disease, which would you choose, the
one
that may increase viral load or the one that does not? Another possibly
practical benefit of our research! How is it that diferent
liposomal
forms produce different responses? It is likely some combination of
AmB-induced
membrane potential changes, specific ion currents, calcium fluxes or
TLR receptor activation.
That
is a subject of ongoing research.
In another collaboration wiuth Dr. David Lewis at UW-EC, we are
trying to develop new fluoresccent probes and fluorescent tags for
Amphotericin.
We are currently comparing a naphthalamide probe which can distribute
into
acidic organelles like lysosomes and the Golgi apparatus. It functions
much the the commercial
Lysotrackertm probes from Molecular
Probes.
Hence we refer to this probe as InstantLyso.
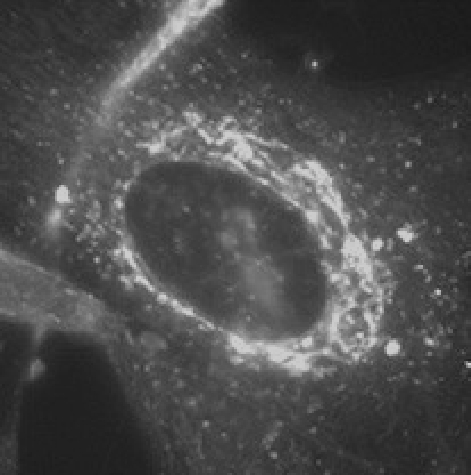
Images of InstantLyso and other dyes.
An epifluorescence image of InstantLyso with live fibroblasts
at 75 nM and excited with blue filter light (~460-490 nm WIB set).
Notice the clearly visible Golgi apparatus which is particularly
targeted in this cell line.
We have also developed fluorescent stains for cholesterol rich
domains (InstantLipo) which may also effecitvel stain so-called 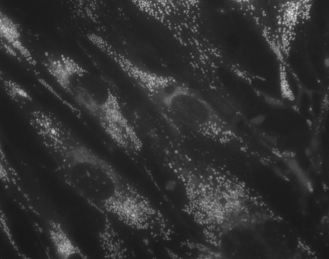 lipid "rafts" and pathological cholesterol inclusions.
This probe may be useful for diagnosing cholesterol and other lipid
disorders.
lipid "rafts" and pathological cholesterol inclusions.
This probe may be useful for diagnosing cholesterol and other lipid
disorders.
Most interesting are the totally novel mitochondrial
stains developed here at UW-EC. 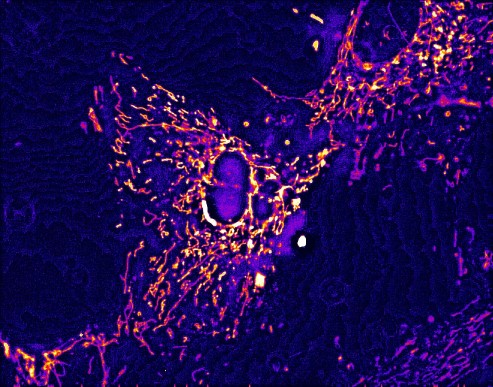 A photo of the
filamentous mtiochondria in fibroblasts is shown below. All photos were
imaged
in the fluorescence microscope facility at UW-Eau Claire.
A photo of the
filamentous mtiochondria in fibroblasts is shown below. All photos were
imaged
in the fluorescence microscope facility at UW-Eau Claire.
A recent very exciting area of research has come from
collaboration with Alan Dispirito of Iowa State University. We are studying a peptide, Methanobactin,
that strongly binds and reduces metals in the environment, especially
copper (see these papers, 10
and 11
for more detail). It can also reduce Au(III) to gold nanoparticles.
This molecule could have major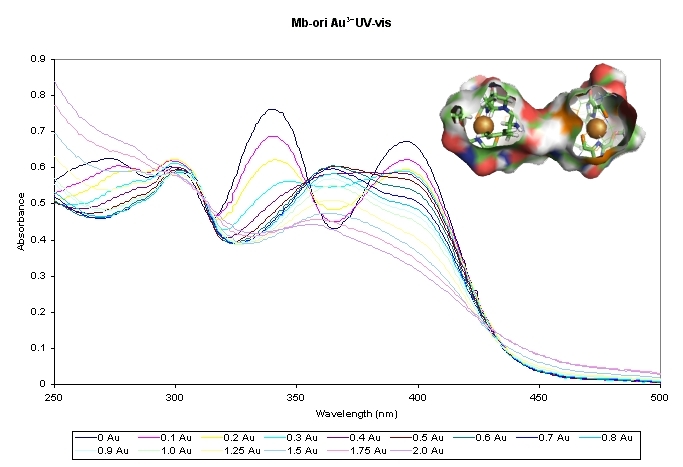 impacts on
weathering of minerals and mobilization of toxic metals in the
environment. We are beginning 1 and 2D NMR studies on the
structure of this peptide-like molecule.
impacts on
weathering of minerals and mobilization of toxic metals in the
environment. We are beginning 1 and 2D NMR studies on the
structure of this peptide-like molecule.