Function of Triosephosphate Isomerase
The function of TIM is the reversible interconversion of dihydroxyacetone phosphate (DHAP) and glyceraldehyde-3-phosphate (GAP) (i). The fourth step of glycolysis is the aldol cleavage of fructose 1,6-bisphosphate in to DHAP and GAP. GAP can go directly on to the next reaction however DHAP must under go a isomerization to form GAP. This is done by TIM, TIM also can convert GAP to DHAP (viii). Although the roll of TIM is important it is not a control point of glycolysis. The control points before TIM are hexokinace and phosphofructokinase while pyruvate kinase is a control point after TIM (iii). The mechanism for this reaction has four steps and is reversible. The mechanism will be described from DHAP to GAP.. (step 1) After binding of DHAP one of the C-1 protons (1-pro-R) is abstracted by the catalytic base Glu 165, yielding a corresponding enediolate species. (step 2) This enediolate is then protonated at O-2 by the neutral imidazole ring of His 95, giving a doubly protonated enediol and an imidazolate anion. (step 3) His 95 recaptures a proton from the O-1 oxygen again yielding an enediolate species, now lacking the proton at O-1. (step 4) Protonation of this enediolate at C-2 by the carboxyl group of Glu 165, thus restoring the general base to give the product D-GAP, which then is released from the enzyme (v).
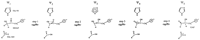 Click here to see the big picture.
Click here to see the big picture.
Forward to Structural Anatomy of TIM
Back to Introduction
Back to Table of Contents
Created by Jason Hadrath
hadratjm@UWEC.edu
Student of Dr. W. Gallagher
at the University of Wisconsin at Eau Claire
Chemistry Department
![[UWEC Web]](../Images/smuwec.gif) ..[Chemistry Dept.]
..[Chemistry Dept.]