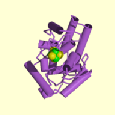
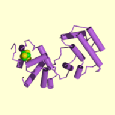




E. coli endonuclease III is a 23 kDa protein and contains 211 amino acid residues. It is elongated and bilobal with a deep cleft separating two similarly sized globular domains. One domain has 111 residues and the other has 100 residues. Both domains have extensive interior hydrophobic cores and polarly charged surfaces suggesting the enzyme is unlikely to form dimers or larger multimers. The enzyme is monomeric. The two domains are known as the 6-helix barrel and the [4Fe-4S] cluster (6). The secondary structure is primarily alpha-helical and the helices are sequentially lettered alpha A-alpha J (11).
The 6-helix barrel domain is formed by six antiparallel helices, alpha B through alpha G. The packing of alpha B through alpha E resembles a standard four-helix bundle. The 6-helix bundle has an unusual overall right handed twist, unlike the left handed twist of most helix bundles. This right-handed twist and the large angles resemble a helical version of a beta-barrel (6).
The [4Fe-4S] domain is a four-helix domain. Based on its topology, which has a +2 connection between both helix pairs alpha A-alpha H and alpha I-alpha J, this domain is a Greek-key helix bundle. The [4Fe-4S] cluster is ligated by cysteins 187,194, 197, and 203 in an unusually short (17 residue) sequence. The ligand spacing is Cys-X6-Cys-X2-Cys-X5-Cys, which is unlike other [4Fe-4S] proteins (6).
Click here to see a picture of the [4Fe-4S] cluster and its ligand Cys residues. [13]
| Click on the buttons to change image: |
[Introduction][Function][DNA Interactions][References]
Rachel Meyer
Department of Chemistry
meyerrt@uwec.edu
![]() Note: If you can't see the image in the black frame, you have to download the plug-in CHIME by pressing the button above. The script buttons work for Mac's, PC's or SGI's with Netscape 3.0x or later. Netscape 4 sometimes has problems.
Note: If you can't see the image in the black frame, you have to download the plug-in CHIME by pressing the button above. The script buttons work for Mac's, PC's or SGI's with Netscape 3.0x or later. Netscape 4 sometimes has problems.