Introduction to Cytosolic Phospholipase A2:
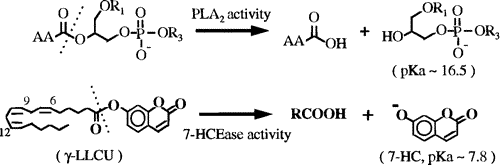
Cytosolic Phospholipase A2 (cPLA2) is an enzyme which plays a significant role in the liberation of arachidonic acid form the sn-2 position of cellular phosholipids in most, if not all, mammalian cells. cPLA2 also has 7-hydroxycoumarin esterase (7-HCEase) activity, which will not be discussed in this mini-review.

The released arachadonic acid is used in many cases for the biosynthesis of eicosanoids, prostaglandins, leukotrienes, and thromboxanes. All of these biological molecules play an important part in many aspects of cell life including proliferation and the infamatory response. The enzyme itself consists of a catalytic domain joined to a membrane binding, N-terminal, calcium-dependant domain, also known as the C2 domain. It is activated by sub-micromolar concentrations of Ca2+ ions and also by phosphorylation of several amino acid residues by mitogen-activated protein kinases (MAP kinases). In the Structure section of this mini-review, for simplicity, these two seperated domains will be discussed seperately.
Research indicates that the role of the Calcium ion is in the enzyme-membrane interface activity of the C2 domain. However, Ca2+ is not neccessary for the reaction catalyzed by the enzyme. A recombinant enzyme without the C2 domain will still catalyze the reaction without Ca2+, however, it will not bind to the membrane. The C2 domain can bind to the membrane with appropriate concentrations of Calcium ions without the catalytic domain, and without the subsequent catalysis. A discussion of the C2 domain will be given in the Structure section of this mini-review.
Other molecules also serve as regulators for this enzyme. Many of these regulators are the intracellular signals which are propogated from cell-surface receptors.
For the most part, the enzyme remains in the cytosol of the cell until it is activated. Once the enzyme is activated, or once the cell is stimulated to release arachidoate, cPLA2 translocates to the nuclear envelope, possibly the endoplasmic reticulum and/or the Golgi, and also vessicles within the cell.
Home Structure Binding Conclusion/References
W. D. Keeton, III
University of Wisconsin -- Eau Claire
Biochemistry/Molecular Biology