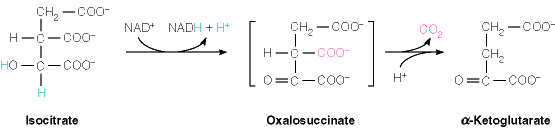
Isocitrate dehydrogenase (IDH) is an important enzyme in the tricarboxylic acid cycle, which occurs in the mitochondrial matrix. IDH is responsible for catalyzing the reversible conversion of isocitrate to alpha-ketoglutarate and CO2 in a two-step reaction (14). The first step of the reaction involves the oxidation of isocitrate to the intermediate oxalosuccinate. The second step of the reaction looses the beta-carboxylate of the oxalosuccinate intermediate as carbon dioxide leaving alpha-ketogluterate (6). During the catalyzation of isocitrate to alpha ketoglutarate either NADH or NADPH is produced along with carbon dioxide. NAD+ and NADP+ are reduced to NADH and NADPH. Their role is to act as hydrogen acceptors from C2 of isocitrate, during the catalysis of isocitrate to alpha-ketoglutarate (7). IDH is dependent on NADP+ and usually on the bound metal Mg2+, but Ca2+ could also be the bound metal. Calcium has been shown to bind to IDH as a complex with isocitrate, acting as a competitive inhibitor of magnesium. With calcium in the active site the turnover rate is reduced because, the isocitrate, enzyme, NADP+, and Ca2+ are shifted in the active site by one angstrom to accommodate the calcium, which is larger than magnesium (14). The magnesium cofactor is involved in stabilizing the transitional states during the two-step reaction (6).
Figure 1: Likely mechanism for the reaction catalyzed by IDH
IDH is a homodimer of 416 residues with a molecular weight of 45.7 kD. The structure of IDH is composed of fourteen alpha helices and eighteen beta sheets. The alpha helices are found all over the structure, whereas the beta sheets, which include parallel and anti-parallel; are found mainly through the center of the structure (18). The standard free energy change for the conversion of isocitrate to alpha-ketoglutarate is –8.4 kJ/mol or –2.0 kcal/mol (15). This spontaneous reaction is driven by the reduction of NAD+ to NADH.
JMol Image of Isocitrate Dehydrogenase (1HJ6)
| IDH is shown above colored by domain. The small domain is colored blue, whereas the large domain is colored orange. NADP+ and Mg+2 colored by atom. Mg+2 is colored green. The loop that binds NADP+ to the active site is colored a darker green. |
Due to IDH's large negative free energy change, it is one of the irreversible reactions in the TCA cycle, and therefore must be carefully regulated to avoid unnecessary depletion of isocitrate (20). IDH is allosterically regulated positively by ADP in mammals and inhibited by ATP, NADPH, or NADH (7). By allosterically regulating IDH, the enzyme will not catalyze its reaction unless levels of ADP are low. If ATP, NADPH, or NADH levels are high then IDH will not catalyze its reaction because there are already ample amounts of the needed product from TCA cycle to go into other cycles (15).
IDH consists of two domains. The small domain is similar to the standard Rossmann fold found in many dehydrogenases. However, the larger domain binds at the interdomain cleft and is associated with two strands of anti-parallel beta-sheets. Therefore, there is no interaction between the enzyme and the hydroxyl of the adenine ribose. The adenine ribose and pyrophosphate backbone are weakly bound and exposed to solvent in IDH (14). The Mg2+-isocitrate complex, is bound between the large and small pockets of IDH, and occurs during the two-step catalysis of isocitrate to alpha-ketoglutarate. Potential hydrogen bonds are formed between isocitrate and Ser 113 residue, Arg 119, 129, 153, Tyr 160, Lys 230, and five water molecules. NADP+ binds between the same cleft as isocitrate, between the large and small domains of the enzyme, and above isocitrate. The sidechains of Ile 37, 320, His 339, Ala 342, and Val 351, the aliphatic portion of sidechains Asn 352 and Asp 392 and the main chain at residues Gly 321 and Asn 352 form the binding site for the adenine moiety (6).
In the forward reaction, Mg2+ binds to isocitrate, which binds to the active site of IDH via it’s free carboxylate group and the residues: Arg 119, 129, Asp 307, 311, Ser 113, and Tyr 160 (14). The first step is isocitrate C2 hydroxyl group’s proton being oxidized to base Asp 283, and the transfer of C2 hydride to NADP+ to form oxalosuccinate and NADPH. This step is regulated by Ser 113, which inhibits both the binding of isocitrate and the proper orientation of the nicotinamide ring for a hydride transfer to take place. When IDH is phosphorylated by Ser 113 residue, the enzyme is completely inactivated via direct electrostatic and steric interactions between the phosphate oxygens and the gamma-carboxylate of isocitrate (14).
NADP+ binds to the active site in the interdomain cleft. The interactions with isocitrate and magnesium help to align the nicotinamide ring of NADP+ so a hydride transfer can occur. Residues Tyr 345, 391, Arg 395, and Asn 352 interact with NADP+ to bind it to the active site (8).
The product of the first reaction is the intermediate oxalosuccinate. Magnesium plays an important role in stabilizing this intermediate by forming an octahedral arrangement of isocitrate, Asp 238, 307, and water molecules (8). The oxygens of alpha carboxylate and isocirates C2 hydroxyl group align the magnesium ion. The position of Mg2+ is also used to stabilize the negative charge formed on the hydroxyl oxygen during decarboxylation. In the second reaction, the beta carboxylate of oxalosuccinate is lost as carbon dioxide, and is followed by Tyr 160 and Lys 230, which serve as acid catalysts that protonate the beta carbon after decarboxylation forming alpha ketoglutarate (6).