Transferases are a class of enzymes responsible for catalyzing reactions where groups of atoms are moved from one molecule to another. These enzymes are used in a variety of different organisms to accomplish a vast array of goals. DNA methyltransferase and acyltransferase are responsible for gene regulation in eukaryotes. Glutathione transferases play a major role in the detoxification of substances by adding a molecule to the toxin, rendering it less harmful and allowing for easier excretion from the organism. They also play an important role in prostaglandin and steroid hormone synthesis. When tissues are injured, Ecto-ADP-ribose transferases are used to transfer ADP-ribose from NAD(+) to arginine residues on cell surfaces to assist in initiating the tissue repair response.
In general, a transferase catalyzed reaction looks something like the following;
AX + B 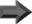 A + BX
A + BX
In this straightforward example, molecule X would be the group transferred by the enzyme, molecule A would be the donor group, and molecule B would be the acceptor group. Most reactions are not this simple, however, and can require several steps before the donated molecule completes transfer. Also, many different mechanisms for this reaction exist depending on the type of transfer taking place.
This project focuses on two enzymes found in the bacterium Escherichia coli – an aminotransferase and phosphotransferase – and one instance where E. coli was used to clone the enzyme – a sulfurtransferase – found in the organism Leishmania major, a parasite that attacks intracellular components of the immune system, such as macrophages and dendritic cells. These enzymes are responsible for various activities. Although they occupy the same family of enzymes and have some similarities in structure and function, these enzymes also have marked differences between them, and share less than 20% sequence homology with one another.