Universal Carrier of Acyl Groups
COA:
Coenzyme A is the universal carrier for acyl groups. Much like citrate transports Acetyl COA across the mitochondrial barrier, COA transports acyl groups to reactions and provides thermal stability and a high energy bond for catalysis.
Here is the molecular structure of COA:
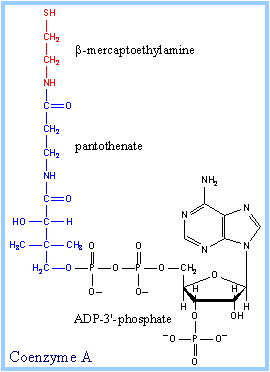
backbone of COA
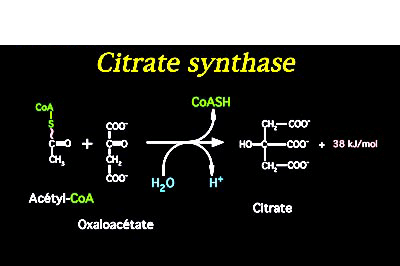
Here is a reaction whereby COA provides energy through its thiol ester bond:
Succinyl)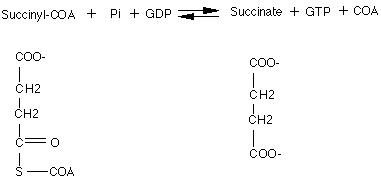
COA-Citrate Synthase is an example of the next feature of COA. It is a very heat stable molecule. In fact it is present and functional in the entire temperature range that life exists (studied from Antarctic bacterium and Archaeon Pyrococcus furiosus).
Antarctic bacterium:
A comparison structurally in COA-Citrate Synthase from the cold to a more thermophilic host, the cold-active enzyme has a much more accessible active site, an unusual electrostatic potential distribution and an increased relative flexibility of the small domain compared to the large domain. All this allows for easier access to the active sites for slower moving substrates. Some other features of cold active enzymes are: reduced subunit interface interactions with no inter subunit ion-pair networks; loops of increased length carrying more charge and fewer proline residues; an increase in solvent-exposed hydrophobic residues; and an increase in intramolecular ion pairs. By lowering the amounts of residues binding each other and solvent, and widening the loops and active sites the protein is more able to resist constriction by lower thermal states.
Archaeon Pyrococcus furiosus:
For hyperthermophiles, thermal denaturation citrate synthase appears to be resisted by complex networks of ion pairs at the dimer interface, a feature common to other hyperthermophilic proteins. Just the opposite of cold proteins, the hot proteins have increased intrastructural binding to keep it from denaturing.
IN HUMANS
Humans use the same citrate synthase in the production of Fatty Acids:
Acetyl groups are necessary components of fatty acid synthesis. For example, in the human synthesis of Palmitate, eight molecules of acetyl COA are required along with 14 molecules of NADPH and 7 ATPs. One problem in Palmitate synthesis lies in the production logistics. Palmitate is synthesized in the cytosol whereas its building blocks (Acetyl COA) is synthesized in the mitochondria. Normally molecules would diffuse through the mitochondrial membrane, however it is impermeable to bare Acetyl COA. The answer to this transportation problem is the condensation of acetyl COA and Oxaloacetate to form Citrate. Citrate readily passes through the mitochondrial membrane and hydrolysis on the cytosol side releases the acetyl COA for use in fatty acid synthesis. Palmitoyl
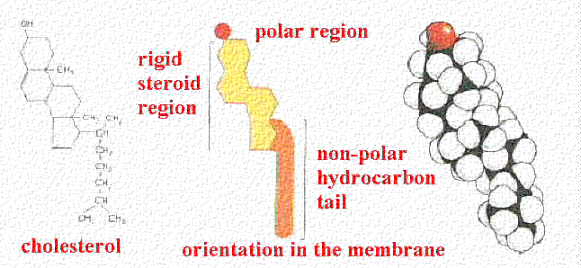
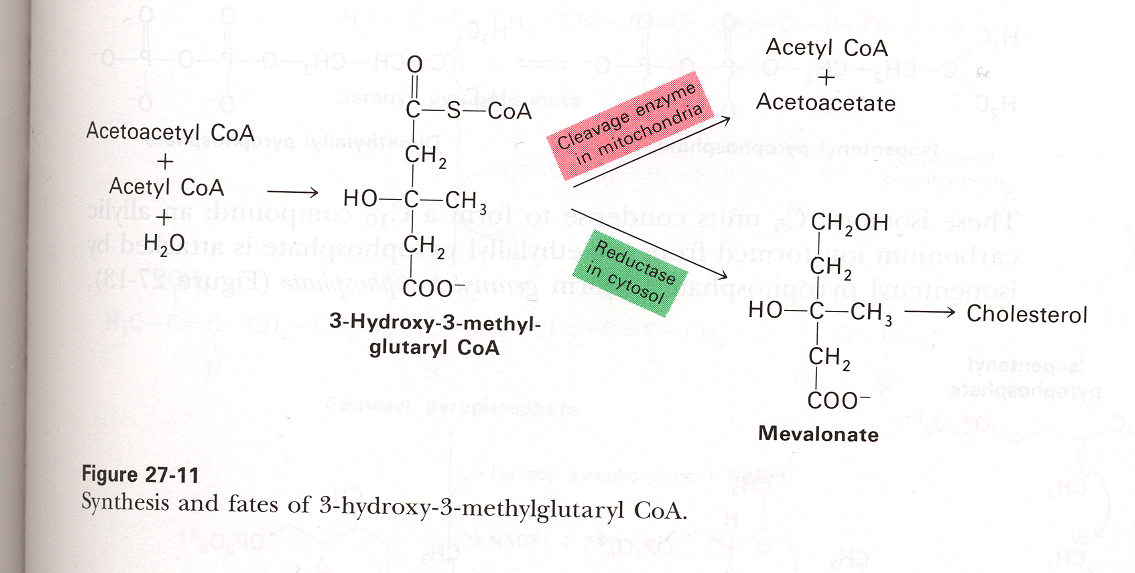
Production of Cholesterol:
Cholesterol is a molecule synthesized from Acetyl COA. It is involved in maintaining the fluidity of Eukaryotic membranes, and as a precursor to many steroid hormones like progesterone, testosterone, estradiol and cortisol. In fact all 27 carbon atoms of cholesterol are derived from Acetyl-COA. Here is a diagram of how Cholesterol inserts into the membrane along with its spacefill model.