Iron-Sulfur Clusters
OTHER IRON-SULFUR ENZYMES INVOLVED IN ETS
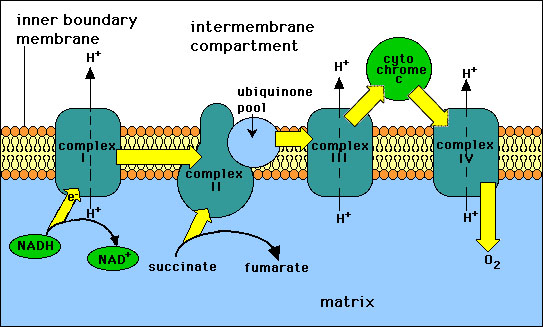
Succinate dehydrogenase is not the only complex in that contains iron-sulfur clusters. In fact, there are two other iron-sulfur containing complexes in the respiratory chain. In the respiratory chain electrons are passed from NADH to Oxygen through a series of 3 large protein complexes called NADH reductase (Complex 1), cytochrome reductase (Complex II), and cytochrome oxidase (Complex III). the electron flow through these transmembrane complexes lead to the pumping of protons across the inner mitochondrial membrane. These complexes utilize flavins, iron-sulfur clusters, hemes, and copper ions as the electron carrying groups. Electrons are passed from NADH reductase to reduced ubiquinone to cytochrome reductase. Ubiquinone is a hydrophobic quinone that diffuses rapidly with in the inner mitochondrial membrane. Ubiquinone also carries electrons from FADH2 that are produced from the oxidation of succinate in The Citric Acid Cycle to cytochrome reductase. Cytochrome c carries electrons from cytochrome reductase to cytochrome oxidase. Cytochrome oxidase is the final complex in the chain. Oxygen is the final electron acceptor. The succinate dehydrogenase is the only complex that does not pump protons.
In the last 5 to 10 years several advances have been made on understanding the structure, function, and properties of Fe/S clusters. Fe/S clusters are not limited to electron transfer and are known to have a vulnerability to oxidation state of the Fe/S cluster and can act as a reversible switch. "It has been found that Fe/S clusters are speculated to have the ability to mediate two electron redox processes, coupled proton and electron transfer, and catalyze disulfide reduction and reductive cleavage of S-indenosylmethionine through the use of sulfur-based cluster chemistry.
Iron sulfur clusters