In humans, biotin is involved in important metabolic pathways such as gluconeogenesis, fatty acid synthesis, and amino acid catabolism. Biotin regulates the catabolic enzyme propionyl-CoA carboxylase at the posttranscriptional level whereas the holo-carboxylase synthetase is regulated at the transcriptional level.
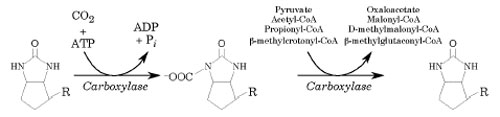
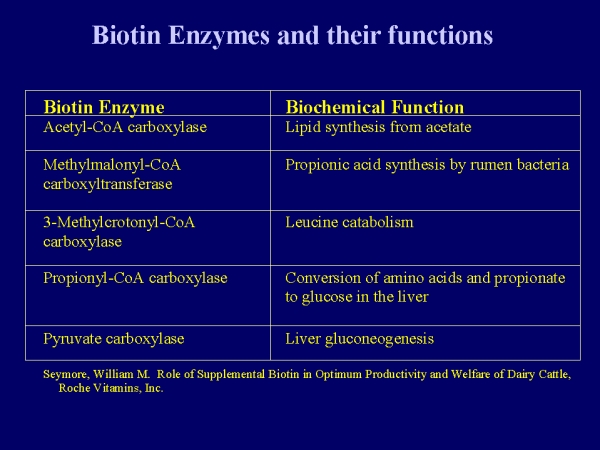
Biotin functions as a cofactor that aids in the transfer of CO2 groups to various target macromolecules. Biotin has nine host enzymes with which it is associated. Humans only have four of these enzymes:
- Pyruvate carboxylase (formation of oxaloacetate from pyruvate)
- beta-Methylcrotonyl-CoA carboxylase
- Propionyl-CoA carboxylase (conversion of propionyl-CoA to succinyl-CoA)
- Acetyl-CoA carboxylase (carboxylation of acetyl-CoA to malonyl-CoA)
Biotin's other target enzymes include Steptividin, Avidin, homocitrate synthetase, and isopropylmalate synthase.
In order to provide glucose for vital functions such as the metabolism of RBC's and the CNS during periods of fasting (greater than about 8 hrs after food absorption in humans), the body needs a way to synthesis glucose from precursors such as pyruvate and amino acids. This process is referred to as gluconeogenesis. It occurs in the liver and in kidney. Most of Glycolysis can be used in this process since most glycolytic enzymes are reversible. However three irreversible enzymes must be bypassed in gluconeogenesis vs. glycolysis: Hexokinase, Phosphofructokinase, and Pyruvate kinase. Phosphofructokinase, and/or hexokinase must also be bypassed in converting other hexoses to glucose. Let's begin with pyruvate. How is pyruvate converted to PEP without using the pyruvate kinase reaction? Formally, pyruvate is first converted to oxaloacetate, which is in turn converted to PEP. In the first reaction of this process Pyruvate carboxylase adds carbon dioxide to pyruvate with the expenditure of one ATP equivalent of energy. Biotin, a carboxyl-group transfer cofactor in animals, is required by this enzyme:
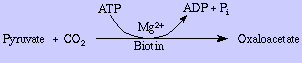
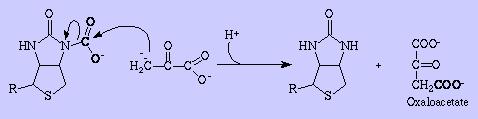
To demonstrate how biotin fuctions as a cofactor, let's uses Pyruvate carboxylase as an example. Carboxylases are synthesized as apo-carboxylases without biotin and the active form is produced by their covalent binding of biotin to the epsilon-amino group of a lysine residue of the apocarboxylases.
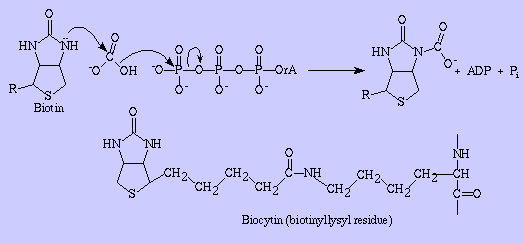
This reaction is catalyzed by the holo-carboxylase synthetase. The last step in the degradation of carboxylases, the cleavage of the biotinyl moiety from the epsilon-amino group lysine residues, is catalyzed by biotinidase and results in the release of free biotin, which can be recycled.