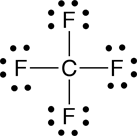
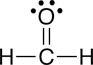
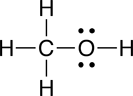
Understanding why molecules are attracted to some molecules but not to others is very important for understanding biological systems at the molecular level. For example, it is these interactions that can be used to explain why ethanol (ethyl alcohol) can dissolve in water, whereas corn oil does not; ethanol is attracted to water molecules whereas corn oil molecules are not. Similar competing interactions with water are what are responsible for the formation of many of the structures found in a living cell, such as membranes and functional proteins. The interactions between molecules that lead to these favorable and unfavorable interactions are collectively referred to as noncovalent interactions.
Most of the non-covalent interactions arise from electrical attractions and repulsions. This is the case, even though molecules, as we have learned, have no net charge. This is because for some molecules, the electrons are not evenly distributed in molecule relative to the protons. This leads to positively and negatively charged regions within a molecule. Figure 1, below, shows with an ethanol molecule.
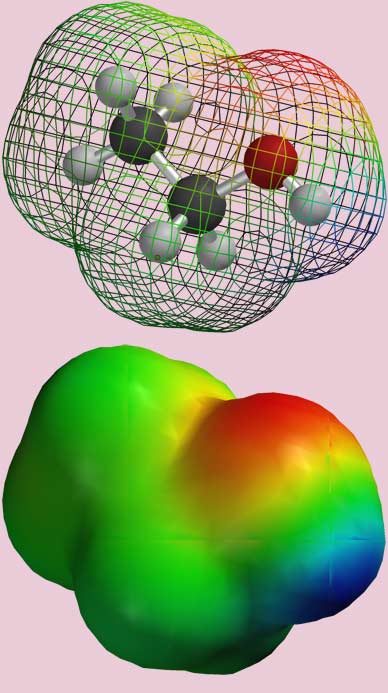 |
Calculating the distribution of electrons in a molecule is very complicated and goes way beyond what we can expect do in this class, however, there are some simple rules that can be applied to predict in general terms, the polarity of a molecule. There are basically three steps involved and these are discussed in Section 4.2 of Raymond.
Identifying Polar Covalent Bonds
The first step is to identify any polar covalent bonds in the molecule. Polar covalent bonds arise because the different elements do not share the bonding electrons equally; the more electronegative an element is, the more it pulls the bonding electrons to its side of the bond and the more negative that side of the bond becomes. Each element has been assigned an electronegativity values and these are shown in Figure 2.
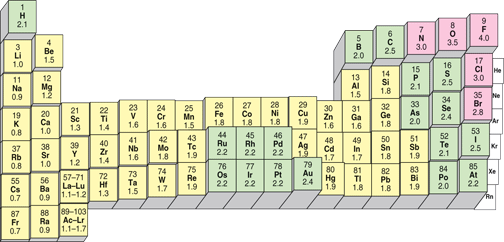 |
These values range from 0.7 to 4. In general, the metals have electronegativity values less than 2, and non-metals have values greater than 2. When two elements combine which have electronegativity values that differ from one another by more than 2, then the more electronegative element takes the valence electrons away from the less electronegative element and an ionic bond is formed between them. This is why metals and non-metals combine to form ionic compounds. When the difference in electronegativity is less than 2, then a covalent bond is formed. This happens when two different non-metals combine to form a covalent bond. When the difference is small or zero, the bond is considered nonpolar. When the difference approaches 2, the bond is considered a polar covalent bond. This is summarized below in Figure 3.
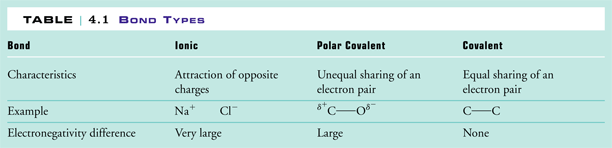 |
For our purposes, the following rules-of-thumb can be applied
- Metals bonding to nonmetals will form ionic bonds
- Nonmetals bonding to nonmetals will form covalent bonds
- If the bond is between either a carbon (C) or a hydrogen (H) and a nitrogen (N), Oxygen (O), Fluorine (F) or Chlorine (Cl), the bond is polar covalent
- All other covalent bonds are nonpolar covalent.
If a molecule contains no polar covalent bonds, then it will be a nonpolar molecule and you need to go no further. If the molecule contains only one polar covalent bond, then it will be a polar molecule, and you also need to go no further. If, however, the molecule contains more than one polar covalent bond, then you next have to determine the shape of the molecule to see if the polar covalent bonds cancel one another out or not.
Determining the Shape of a Molecule
The shape of a molecule is determined in two steps. First, you identify how many groups of electrons surround the central atom on a molecule, this would be one of the atoms participating in a polar covalent bond. A group of electrons is defined as one of the following:
- A single bond
- A double bond (this is considered one group of electrons)
- A triple bond (this is also considered on group of electrons)
- A non-bonded pair of electrons.
The number of groups of electrons will determine the geometry about the central atoms:
- 4 groups: tetrahedral geometry
- 3 groups: triangular geometry
- 2 groups: linear geometry
After determining the geometry, the shape is determined by looking at just the bonds to the central atom and ignoring the non-bonding pairs of electrons. The possible shapes are listed in Table 4.2 in Raymond.
Determining the Polarity of Molecules Containing Polar Covalent Bonds
After determining the shape of a molecule containing polar covalent bonds, look to see if the polar bonds are arranged symmetrically such that they cancel each other out. If they do not, then the molecule is polar. This is often done by using arrows to represent the polar bonds, with the arrows pointing from the positive to the negative end of the polar bond. If the arrows are viewed as forces that are either pushing or pulling on the molecule, the molecule is polar if those forces would add together and cause the molecule to move. If the molecule is nonpolar, the forces will cancel each other out and the molecule stays put. This is illustrated in Figure 4, with methylene chloride, which is a polar molecule, and carbon dioxide, which is a nonpolar molecule that contains polar bonds.
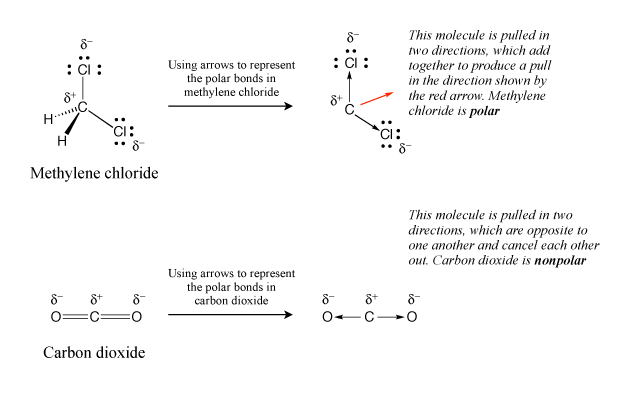 |
Putting It All Together
Figure 5 below contains a flowchart that illustrates the steps used to determine if the molecule is polar. The examples shown are from Figure 4.7 in Raymond.
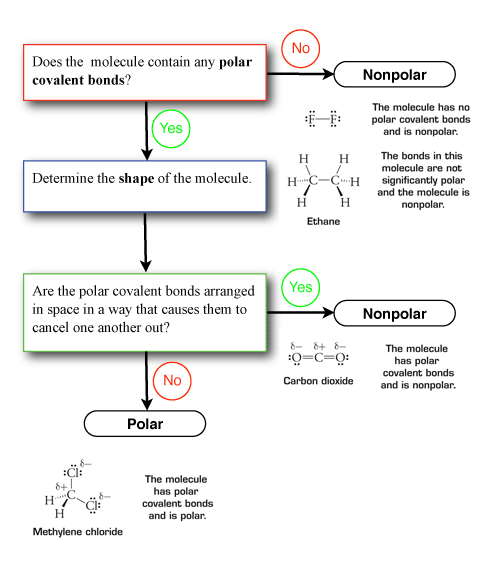 |