Which of the following molecules is polar?
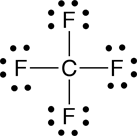 a. carbon tetrafluoride |
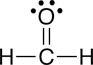 b. formaldehyde |
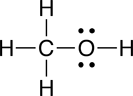 c. methanol |
The carbon tetrafluoride is nonpolar, where as formaldehyde and methanol are polar.
Applying the scheme that is summarized in Figure 5, each of these molecules contains at least one polar bond. This is because each contain at least one of the strongly electronegative atoms (nitrogen, N; oxygen, O; fluorine, F; or chlorine, Cl), that is covalent bonded to another non-metal atom.
Looking next at the shapes of these molecules
a. The geometry about the carbon (C) in carbon tetrafluoride is tetrahedral (four groups of electrons). Since there are no non-bonded pairs of electrons on the carbon, the shape is also tetrahedral (See Table 4.2 in Raymond).
|
|
When arrows are used to represent the polar bonds in this molecule, we see that the polar bonds cancel one another out. Therefore, carbon tetrafluoride is nonpolar.
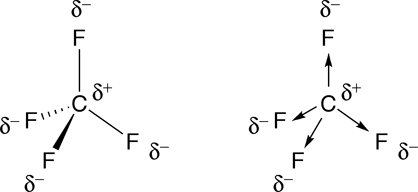 |
b. The geometry about the carbon (C) in formaldehyde is triangular (three groups of electrons). Since there are no non-bonded pairs of electrons on the carbon, the shape is trigonal planar (See Table 4.2 in Raymond).
|
|
When arrows are used to represent the polar bonds in this molecule, we see that there is only one polar bond. Therefore, formaldehyde is polar.
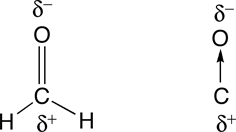 |
c. The oxygen in methanol is involved in two polar bonds, so we will focus on the geometry about this atom. The geometry about the oxygen (O) in methanol is tetrahedral (four groups of electrons). Two of these groups of electrons are the non-bonded pairs of electrons on the oxygen, so the shape is bent (See Table 4.2 in Raymond).
|
|
When arrows are used to represent the polar bonds in this molecule, we see that there are two polar bonds which do not cancel one another out, therefore, methanol is polar.
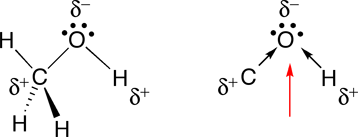 |