You book defines a compound as matter constructed of two or more chemically combined elements. In this course we will encounter two types of compounds, ionic compounds and molecular compounds. Ionic compounds are the subject of Section 3.4 in Raymond. Ionic compounds are made up of two types of ionic species; cations, which are positively charged, and anions, which are negatively charged. Ionic compounds are are held together by ionic bonds, which is essentially arises from the electrical attraction that positive and negative ions have for each other. Ions can form from individual atoms of an element; these are called monoatomic ions. As was discussed in Section 3.3 of Raymond and in the Elaboration - The Octet Rule, the charge on a monoatomic ion formed from one of the representative elements can be predicted using the octet rule.
Molecular compounds are the subject of Sections 3.5 and 3.6 in Raymond. Molecular compounds contain groups of atoms that are held together by covalent bonds. Covalent bonds form between non-metal atoms as and alternative way of obeying the octet rule. Since all nonmetals are looking to gain electrons, when they interact directly with themselves they are unable to gain and lose electrons to form ions. Instead, they share pairs of valence electrons and each atom counts the shared electrons as part of their valence shell. An example is ammonia, which has a formula of NH3. Both nitrogen and hydrogen are non-metals. The electron dot structure's for nitrogen and hydrogen are
As can be seen, the nitrogen, which is a member of Group VA, contains 5 valence electron, and the hydrogen, which is a member of Group IA, contains 1 valence electron. Three hydrogens combine with the single nitrogen using three covalent bonds. This can be shown using electron dot structures:
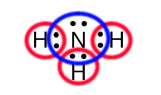
The red circles in this figure include the 2 electrons in the valence shells of each of the three hydrogens. The blue circle in this figure includes the 8 electrons in the valence shell of the nitrogen. The hydrogens and nitrogen are now isoelectronic with an inert gas, the hydrogens with helium and the nitrogen with neon. The pairs of electrons that are included within both a red and blue circle are the bonding electrons. Each pair of bonding electrons is forming a single covalent bond. Later we will see that multiple pairs of bonding electrons can be shared to form double and triple bonds. In ammonia, there is also a pair of electrons on the nitrogen that is not shared with a hydrogen. This pair of electrons is called a non-bonding pair of electrons or a lone pairs.
The formulas for molecules are called molecular formulas. They differ from the formulas for ionic compounds in that they do not just give the ratio of atoms that make up the compound, as is the case for ionic compounds, but instead give the actual numbers of atoms that go to make up the molecules. In a pure molecular substance, all of the molecules are identical, sharing the same molecular formula. For example, here is a model for the solid form of the ionic compound NaCl (Figure 3.13 from Raymond):
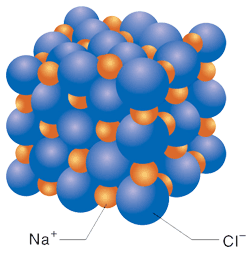 |
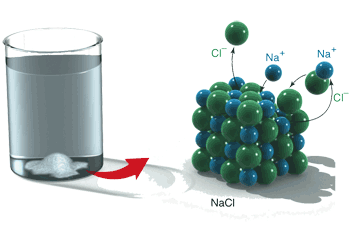
In a solid ionic compound, each ion is surrounded by ions of the opposite charge, but is not associated with any particular ion. There are not molecules in an ionic solid. The formula refers to the ratio of the constituent ions but does not represent the formula for a molecule; it is not a molecular formula. When an ionic solid melts or is dissolved into a solution, the ions separate from one another. Below is a figure that shows what happens when NaCl dissolves in water:
 |
In a molecular compound, the formula is called a molecular formula because it describes the composition of the molecules that make up the substance. The atoms in a molecular substance are associated with specific atoms through covalent bonds. Below is a figure that shows the structure of the molecule ammonia:
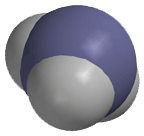 |
The blue sphere represents the nitrogen, while the white sphere represents the hydrogen atoms. The spheres interpenetrate because they are sharing electrons through covalent bonding. When solid ammonia melts or dissolves, the atoms in the ammonia molecules to not separate from one another. An ammonia molecule looks like this whether it is in the solid, liquid or gaseous state. This is very important point that distinguishes ionic compounds from molecular compounds. The ionic bonds that hold ionic compounds together in the solid state are disrupted when the ionic compound melts or dissolves in a solvent. The covalent bonds that hold the molecules in molecular compounds together are not disrupted when a molecular compound melts or dissolves in a solvent. Later we will learn that the forces that hold the molecules together in the solid state, and which are disrupted when a molecular compound melts or is dissolved in a solvent, are called noncovalent interactions. The are generally much weaker than covalent bonds and consequently are the first to be disrupted. These weak, noncovalent interactions are extremely important in biological systems.
There are some groups of atoms that are both molecular and ionic. These are called polyatomic ions. An example the ammonium ion NH4+:
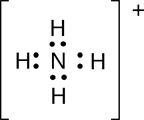
Here there are four hydrogen atoms (H) that are covalently bonded to the nitrogen atom (N) and the overall group of atoms has a 1+ charge, which means there is 1 fewer electron than protons in this species. The brackets are used to indicate that this charge is associated with the entire group of atoms. Later we will learn a way of determining where the positive charge is located in this group of atoms.