To fully appreciate the structure and behavior of atoms on must have a working concept of what the atoms that make up molecules look like. In your introductory chemistry courses you learned that atoms are made up of three types of particles: protons, neutrons and elections. Protons and neutrons have nearly the same mass; but the protons are positively charged, whereas the neutrons are neutral. The electrons, on the other hand, have very little mass compared to the protons and neutrons, and a negative charge that is equal to that of the proton. The atoms for each element is determined by the number of protons it contains, which is indicated by the atomic number for an element. For example, the atomic number for the element carbon (C) is 6, which means that every atom of carbon contains 6 protons. A neutral atom has an equal number of protons and electrons, therefore, a neutral atom of carbon also contains 6 electrons. (For further reading, see Raymond, Sections 2.1 to 2.3)
The mass of an atom is determined primarily by the number of protons and neutrons it contains. The sum of the number of protons and neutrons for an atom is called the atomic mass number. This number is approximately equal to the mass of an atom in atomic mass units. The number of neutrons that an atom of a given element has is variable, which gives rise to different isotopes for an element. For example, the most abundant isotope of carbon has 6 neutrons in addition to its 6 protons, which gives it an atomic mass number of 12. This isotope of carbon is called carbon-12. Other isotopes of carbon that are found in nature include carbon-13, which has 7 neutrons, and carbon-14, which has 8 neutrons. The atomic masses that are given for the elements on the periodic table represent a weighted average of the masses for the different isotopes of an element based on the relative abundance of the different isotopes of an element found in nature.
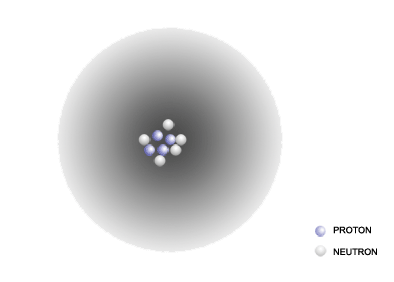
Below is a figure that illustrates this with the three naturally occurring isotopes of hydrogen, hydrogen-1, hydrogen-2 and hydrogen-3:
 |
The labels below each model in this image shows another way of representing the isotopes of an element; a superscript to the left of the chemical symbol for an element indicates the atomic mass number, while a subscript to the left of the chemical symbol indicates the atomic number. The subscript is often omitted, since it is redundant to the chemical symbol; hydrogen (H), by definition, has an atomic number of 1.
The images shown above for hydrogen-1, hydrogen-2 and hydrogen-3 are based on a model for the atom that was developed in the early 1900's. In this model, the protons and the neutrons are tightly packed in the center of the atom in region called the nucleus. The electrons surround the nucleus in a diffuse cloud.
As was described above, the number of protons that an atom contains determines which element it is. The number of neutrons that an atom has determines the isotope of the the element. The number of electrons in an atom can also vary. A neutral atom has an equal number of protons and electrons. This results in the positive charges from the protons being exactly balanced by then negative charges on the electrons. When doing chemistry, atoms are not allowed to gain or lose protons, however, they can gain or lose electrons. This will result in a net charge on the atom. Atoms that have a greater number of electrons than protons, have a net negative charge, such atoms are called negative ions, or anions. Atoms that have a fewer number of electrons than protons, have a net positive charge, such atoms are called positive ions, or cations. For example, calcium (Ca) has an atomic number of 20. This means that all calcium atoms contain 20 protons. A neutral atom of calcium will also have 20 electrons. The most common ionic form of calcium is Ca2+, which has a positive 2 charge. The Ca2+ ion, therefore, contains 18 electrons.