Write the chemical symbol with isotope notation for the element that has the following atomic structure:
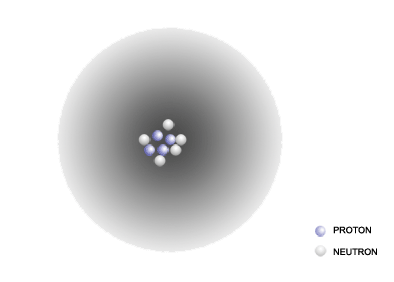
The model of the atom shown in this problem contains 4 protons and 5 neutrons. The atomic number for this element is 4 and the atomic mass number is 9 (4+5). A look at the periodic table reveals that the element with the atomic number of 4 is Beryllium, which has the chemical symbol Be. That makes this atom the beryllium-9 isotope. Putting this all together, the chemical symbol in isotope notation is
![]()
or
![]()
The subscript "4" can be omitted because, by definition, all beryllium atoms have an atomic number of 4.