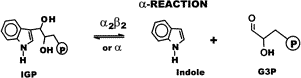 | Tryptophan synthase is an enzyme that converts indole-3-glycerol-phosphate and L-serine to L-tryptophan, H2O, and D-glyceraldehyde-3-phosphate. This reaction is done in two steps, each associated with a different part of the enzyme. The enzyme is a tetramer and is composed of two alpha subunits and two beta subunits. The alpha subunit has the lyase activity and carries out the first half reaction. The alpha subunit performs an aldol cleavage of indol-3-phosphate to indol and glyceraldehydes-3-phosphate. The indol is then transported to the beta subunit for the second half of the reaction. The reaction in the alpha subunit is reversible in the direction described and is favorable. However, the complete reaction of both subunits is irreversible and favorable
There are no cofactors or coenzymes that are required for the alpha subunit reaction to work other than being bound to the beta.
This enzyme is regulated on the genomic level at the trp operon. The exact mechanism of this regulation differs between organisms. |
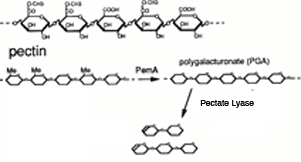 | Pectate lyase is an enzyme that cleaves de-esterfied pectin in plant cell walls. The enzyme will cleave internal glycosidic bond by beta-elimination. This produces oligomers with 4,5-unsaturated residues at the non-reducing end. This reaction is favorable in the forward direction.
There is an important cofactor for this enzyme. Pectate lyase is calcium dependent to induce a conformation change.
Pectate lyase is regulated by calcium ion concentration. Also, because the enzyme works on plant cells and is secreted by bacteria, the rate of secretion can control the rate of the reaction. |
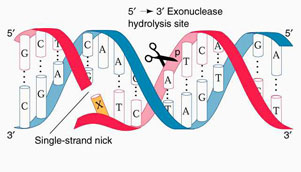 | Apurinic/apyrimidinic endonuclease is a multifunctional protein that has functions ranging from cell cycle control to oxidative signaling. But this enzyme also has an important role in base excision repair. The lyase action comes from the cleaving of the DNA sugar-phosphate backbone at the position that is 5Õ of the apurinic/apyrimidinic base. This reaction is favorable.
Magnesium is a required cofactor for this enzyme to work and two of them associate near the active site.
There are two main regulation factors of apurinic/apyrimidinic endonuclease. The first is the presence of magnesium. The second is the amount of mismatched bases. |