Succinyl-CoA Synthetase Structure
Succinyl-CoA synthetase is a multi-subunit enzyme. It has an alpha and a beta subunit or polypeptide chain (Wolodoko, 1993). Each of these two polypeptide chains then are split up into two different domains. These two domains are named the N-terminal and the C-terminal. Starting with the alpha polypeptide chain the N-terminal and C-terminal is an a/b class protein (SCOP, http://scop.berkeley.edu). This means that this protein is mostly comprised of a mix of alpha helices and beta sheets, mostly parallel sheets. The configuration of the alpha helices and beta sheets are often beta-alpha-beta units. The beta polypeptide chain has some difference between the N-terminal and C-terminal. The C-terminal of the beta polypeptide chain has a secondary structure class much like the N- and C-terminal for the alpha polypeptide chain. It is an a/b class protein with a mix of alpha helices and beta sheets in alpha-beta-alpha units. The difference is found in the N-terminal. It has a secondary structure class of a+b. This means that this terminal is still comprised of alpha helices and beta sheets, but they are generally segregated from one another in different areas of the protein. Also the beta sheets tend to be anti-parallel rather than parallel in a/b class.
The overall enzyme is a alpha2beta2 heterotetramer that has an active unit of alpha-beta. The active site of Succinyl-CoA Synthetase seems to be more complicated than the other examples. In fact there appears to be two active sites each with a Coenzyme A in the active site. There does appear to be some similarities and generalizations about each active site. The histidine 246 in both active sites appears to be a very important residue. This residue is positioned at the end of two alpha helices that are known as the power helices. One of the helices comes from the alpha unit and the second from the beta unit. These two alpha helices stabilize the phosphohistidine in the active site during the reaction. The active site is positioned between these two helices (Wolodoko, 1993).
Succinyl-CoA Synthetase Function
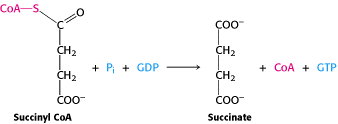
The mechanism of this reaction is rather well documented and understood. First, the coenzyme A thioester bond with succinyl-CoA is broken when orthophosphate bonds with succinyl Co-A, thus making succinyl phosphate. The histidine 246 (referred to above) of the alpha polypeptide chain removes the phosphate group from succinyl phosphate to make phosphohistidine and succinate. This phosphohistidine group (which explained above is stabilized by the power helices) loses its phosphate group when it deviates towards the beta subunit where GDP is bound (sometimes ADP) which then forms the high energy molecule GTP (or ATP) (Berg, 2002). From the mechanism we can see that the reaction from succinyl-CoA to succinate is a very important reaction in the Citric Acid cycle that occurs in the mitochondria. This reaction is very important because in the process of making succinate GTP is formed from GDP. The energy to make this conversion came from the thioester bond that is broken between succinyl and Coenzyme A. GTP is a high energy molecule that can readily be converted to ATP.