Acetyl-CoA Carboxylase Structure
According to the SCOP classification of Baker's yeast (Saccharomyces cerevisiae) the protein class is an alpha and beta protein (a/b). CATH protein structure classification also supported this in saying that it is a class 3 structure which means it has a mix of alpha helices and beta sheets. The beta sheets are mainly composed of parallel beta sheets. These parallel beta sheets are made up of beta-alpha-beta units. The active site of Acetyl-CoA Carboxylase has been characterized and studied in great detail. According to the SCOP classification the Acetyl-CoA Carboxylase falls under the classification of a, “biotin dependent carboxylase carboxyltransferase domain (SCOP, http://scop.mrc-lmb.cam.ac.uk/scop/data/scop.b.d.be.b.e.b.html).” The active site of these biotin dependent carboxylases forms the two different homologous subunits.
The acetyl-CoA carboxylase is made up of three different domains that all work together to produce malonlyl-CoA from acetyl-CoA. The three domains of this enzyme is the biotin carboxylase (BC) domain, the biotin carboxyl carrier protein (BCCP), and the carboxyltransferase (CT) domain. The CT domain is composed of two subdomains, the N and C domains. The N and C domains both are composed of the beta-beta-alpha superhelix back bonefold. The active site is found at the interface of the subdomains (Zhang, 2003). Grover L. Waldrop et al., the author and researcher of Three Dimensional Structure of the Biotin Carboxylase Subunit of Acetyl-CoA Carboxylase, states that, “Those amino acid residues believed to form part of the active site pocket include His 209-Glu 211, His 236-Glu 241, Glu 276, Ile 287-Glu 296, and Arg 338 (Waldrop, 1994).” This amino acid sequence are all part of parallel beta sheets around the active site.
Acetyl-CoA Carboxylase Function
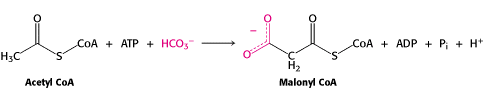
Acetyl CoA Carboxylase plays a significant role in the fatty acid synthesis and degradation in the cytosol. It controls the metabolic cycle of fatty acid synthesis because it is the enzyme that catalyzes the committed step in the production of malonyl-CoA from acetyl-CoA (Zhang, 2003). According to Walrdrop, “The enzyme, found in all animals, plants, and bacteria, catalyzes the biotin-dependent carboxylation of acetyl-CoA to from Malonyl-CoA in a two step reaction mechanism.” The reaction two step mechanism is seen below: (still need to make the graphic of this reaction)
The first reaction which includes the carboxylation of biotin to form carboxybiotin is catalyzed with the biotin subunit of acetyl-CoA carboxylase. This portion of the mechanism is ATP dependent; also the bicarbonate provides the CO2. The second step of this mechanism requires that the carboxyl group be transferred from the biotin to the acetyl-CoA to form malonyl-CoA. This reaction thermodynamically should not be spontaneous, but because it is coupled with the hydrolysis of ATP to ADP this reaction goes. This hydrolysis of ATP also is one main reason this reaction is the committed step of this metabolic cycle. This mechanism is very much like the pyruvate kinase that is a major committed step in glycolysis that converts phosphoenolpyruvate to pyruvate. In the metabolic pathway this enzyme can be allosterically controlled by citrate as seen in the diagram below (Berg, 2002).
