III. Function
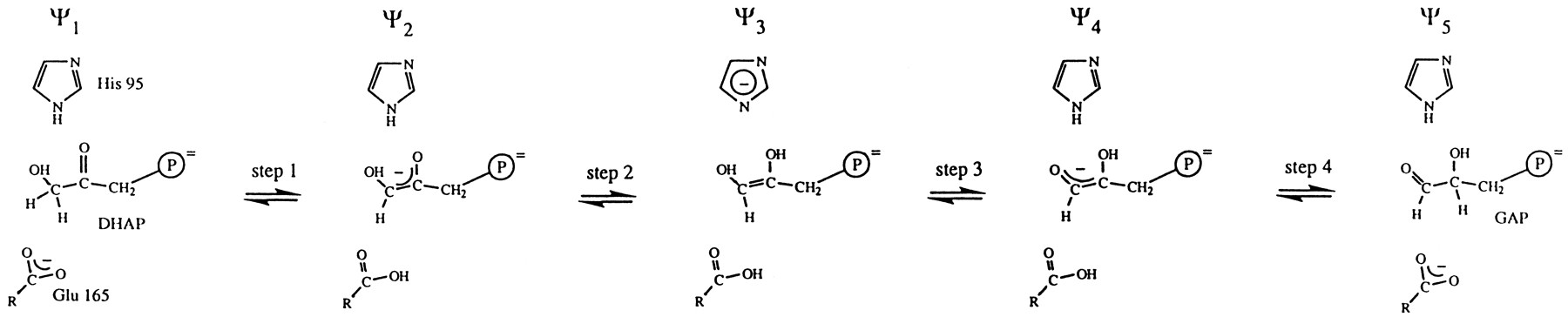
Triosephosphate isomerase reversibly converts DHAP to GAP during glycolysis. This conversion is extremely important for efficient conversion of glucose to energy. If DHAP were not converted to GAP half the energy available from glucose would be lost as an unusable by product. Conversion begins when the Lys12 residue binds the phosphate of the substrate. Then the conversion takes place in four steps. First, Glu 165 abstracts a proton from the substrate at C-1, yielding an enediolate. Second, the enediolate is protonated by the imidazole ring of His 95. This yields a doubly protonated enediol. Third, His 95 abstracts a proton from the O-1 oxygen, returning the substrate to an enediolate. Finally, Glu 165 protonates the enediolate at C-2, producing D-GAP. D-GAP is then released from the enzyme(8).
Triosephosphate is an effiecient catalyst. It can enoliate 2*103 molecuels of DHAP per second. The rate of the reaction is limited only by the avaliability of substrate(9).
Triosephosphate isomerase is not involved in control of the glycolytic pathway. In the steps of glycolysis prior to TIM the control points involve hexokinase and phosphofructokinase. In the steps after TIM control involves Pyruvate kinase(9).
Following is the mechanism of TIM. The steps are:

III. Function