Glucose + 2Pi + 2ADP + 2NAD+![]() 2 pyruvate + 2ATP + 2NADH + 2H+ + 2H2O
2 pyruvate + 2ATP + 2NADH + 2H+ + 2H2O
| Introduction |
Even the most basic forms of life require continual metabolism to survive. The universal form of energy production uses a simple sugar, glucose, to form 2 molecules of adenosine triphosphate (ATP). Glycolysis is the name for the metabolic process that forms these high-energy molecules. Prokaryotes and eukaryotes alike rely on glycolysis. The reason this pathway is universal across all organisms is due to the reaction's independence from oxygen presence and energetically favorable reactions. The simplified chemical reaction of the glycolytic pathway is as follows:
Glucose + 2Pi + 2ADP + 2NAD+![]() 2 pyruvate + 2ATP + 2NADH + 2H+ + 2H2O
2 pyruvate + 2ATP + 2NADH + 2H+ + 2H2O
Glucose is a six carbon molecule, and each pyruvate formed is a three carbon molecule. There is more potential for energy formation by using the NADH for further conversion of pyruvate into lactate or ethanol and CO2 through fermentation. Most eukaryotic organisms use the pyruvate and reduced energy carrier NADH to create more ATP in an oxygen-rich environment. A total of 32 ATP can be formed in advanced eukaryotes if glucose is incorporated into the tricarboxylic acid (TCA) cycle and the reduced energy intermediates are decayed throughout the electron transport chain.
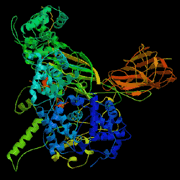
A major step in the breakdown of glucose or a similar monosaccaride into two pyruvates and two ATPs requires pyruvate kinase (PK). PK catalyzes the conversion of phosphoenolpyruvate to pyruvate and ATP by phosphoryl transfer to an ADP-MgII complex and subsequent protonation of the remaining enolate. A monovalent or divalent ion is integral in the completion of this reaction (1). Additionally, the binding of the ADP-MgII to the active site is allosterically regulated by fructose 1,6-bisphosphate (FBP) (2). Glucose regulation is dependent on transcriptional regulation of genes for glycolytic enzymes like PK (3). The reactants and conversion to products are outlined below:
|
|
|
|||||
|
|
|
|
|
||
| Questions? Contact Michael at telisams@uwec.edu |
 |
 |
 |
 |