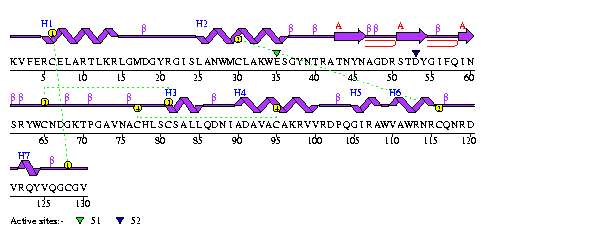
 Figure 5 Sequence of human lysozyme with secondary structure shown. Source: 1LZ1 |
|
Human lysozyme (HL) is made up of 130 amino acids and has a macromolecular weight of 14,691daltons, calculated by electrospray ionization mass spectrometry.[Booth et al., 1997] It is a single strand enzyme with no subunits. The secondary structures of HL can be broken down into seven helices and one beta sheet consisting of three strands. |
To view a close-up of the protein, position cursor on image and press the left mouse button +shift key and then drag mouse. To rotate molecule, move the cursor to picture and then press the mouse button and drag the mouse. Press here to return to a wireframe model. |
 Figure 5 Sequence of human lysozyme with secondary structure shown. Source: 1LZ1 |
| Home | Introduction | Reaction Mechanism | Active Site | Conclusion |