![]()
Regulation
![]()
GPase is a highly regulated allosteric enzyme. This is a result of many different regulatory sites on the enzyme. The net effect of the regulatory site allows the enzyme to operate at a variety of rates; the enzyme is not simply regulated as "on" or "off", but rather it can be thought of being set to operate at an ideal rate based on changing conditions at in the cell.
As stated before, the Ser 14 phosphorylation site is the primary site of GPase activation. This site is phosphorylated as a result of the cAMP cascade. The cAMP cascade is a response to extracellular nervous/hormonal stimulation. Compounds such as epinephrine and glucagon initiate this cascade causing many different intracellular events to occur. One of these events is the activation of the enzyme phosphorylase kinase (PK). PK will phosphorylate GPase at Ser 14. This process activates GPase, allowing it to metabolize glycogen molecules. Another kinase (protein kinase) phosphorylates the enzyme glycogen synthase (GS) suspending the synthesis of glycogen.
This regulation cascade is part of the "fight or flight" response at the cellular level. It is a time when energy usage by the cell is at its maximum. Thus, it is important that the cell have a maximal amount of energy available (primarily for muscle contraction). Inhibiting GS shuts down glycogen synthesis so it is ensured that available glucose will be used for energy production, not storage. GS is inactivated so that it won't cancel the effect of GPase. Once activated, GPase’s activity is regulated to varying extents by a wide variety of small molecules that are themselves involved or are intermediates metabolism.
As previously mentioned, the most important allosteric effector is the phosphate molecule covalently attached to Ser 14. This switches GPase from the b (inactive) state to the a active state. Upon phosphorylation, GPase attains about 80% of its Vmax. When the enzyme is not phosphorylated, GPase activity is practically non-existent at low AMP levels (Zubay 230).
Once phosphorylated, GPase activity can be increased even further with the addition of AMP. AMP is a signal to the cell that energy levels are low and that ATP needs to be made. AMP makes a good signal molecule because it has no "high energy" phosphate ester bonds to break making it unfavorable for energy usage. AMP needs to be added to a concentration of about 60 uM to fully attain Vmax (Zubay 230). AMP can also activate GPase b (the inactive form) to some extent, but it cannot fully activate GPase without phosphorylation. Also, AMP has to be fairly highly concentrated to achieve appreciable activation without phosphorylation. Figure1 is an interesting graph showing the various activation of GPase by phosphate and AMP. Glycogen is also an allosteric effector (Buchbinder et al 22305). This is obvious because if there is a high concentration of glycogen in the cell, it needs to be mobilized instead of taking up more glucose as the cell can only store a finite quantity of glycogen.
Figure 1: Activation of GPase by Phosphorylation and AMP
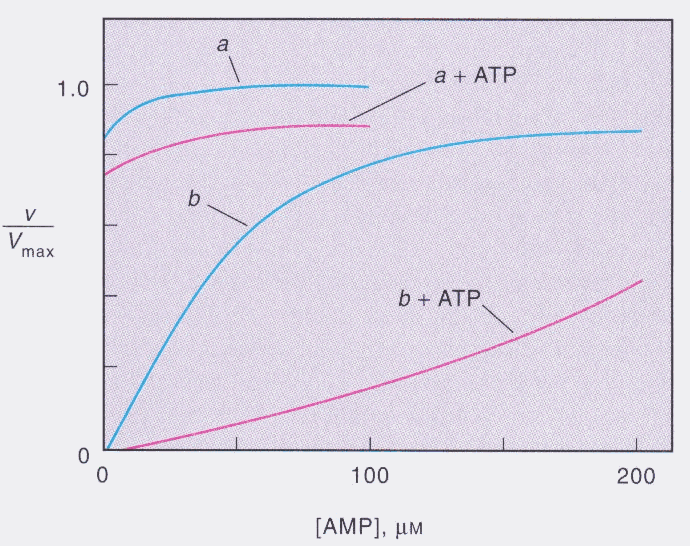
As can be seen in Figure 1, GPase is best
activated by phosphorylation and low levels of AMP (a).
Higher levels of AMP can activate the dephosphorylated
form (b) to high levels. It is obvious though that AMP is
not as an effective allosteric effector alone, but when
coupled with phosphorylation, GPase is fully activated at
relatively low AMP levels (Modified from Zubay 230).
There are many inhibitors of GPase as well. One can follow the same logic about the inhibitors of GPase. The inhibitors are usually metabolites that either carry high-energy bonds, or create molecules that carry high-energy bonds. An example of an inhibitor is ATP, which also binds to the AMP regulatory site (Buchbinder et al 22305). If there is sufficient ATP to bind to and inhibit GPase, then one can assume that there is sufficient ATP in the cell for other cellular functions. If condition arises where more ATP is needed, then the cellular ATP will be used up, and ATP will release from GPase to equilibrate the enzyme bound and solution levels of ATP. When the ATP releases, it is likely AMP will enter the same site and increase the rate of GPase.
Another inhibitor is the molecule G-6-P (Buchbinder et al 22305). G-6-P can be formed from glucose by phosphorylation by hexokinase, or the product of glycogenolysis, G-1-P, can be converted to G-6-P by phosphoglucomutase. If there is enough G-6-P to bind to GPase, then it can be assumed that the cell has enough metabolites available to maintain current energy levels. G-6-P also binds to the AMP site to regulate GPase (Buchbinder et al 22305). G-6-P inhibition is an example of feedback inhibition.
Glucose is competitive inhibitor that binds to the active site of GPase (Buchbinder et al 22305). It also signals that the cell has sufficient fuel to make energy. Glucose also competes with G-1-P for binding to the active site since the phosphorolysis reaction is reversible. While G-1-P can bind to the active site, it is unlikely for a reverse reaction to occur because of the cellular levels of phosphate and G-1-P.
Finally, there is a purine inhibition site (Newgard et al 72). The reason for this site is perhaps a bit more confusing. The common biological purines, adenine and guanine, exist at all times and in various forms in the cell. Adenosine can be in the form of AMP, ADP, or ATP. Guanine can also carry the same forms. Both are also present in DNA and RNA. Molecules such as caffeine or theophylline can also inhibit GPase at this site (Newgard et al 72). At the time of Newgard’s publication (1989) it was not known if there was a physiological ligand that would bind to this site (72).