Reaction Mechanism
![]()
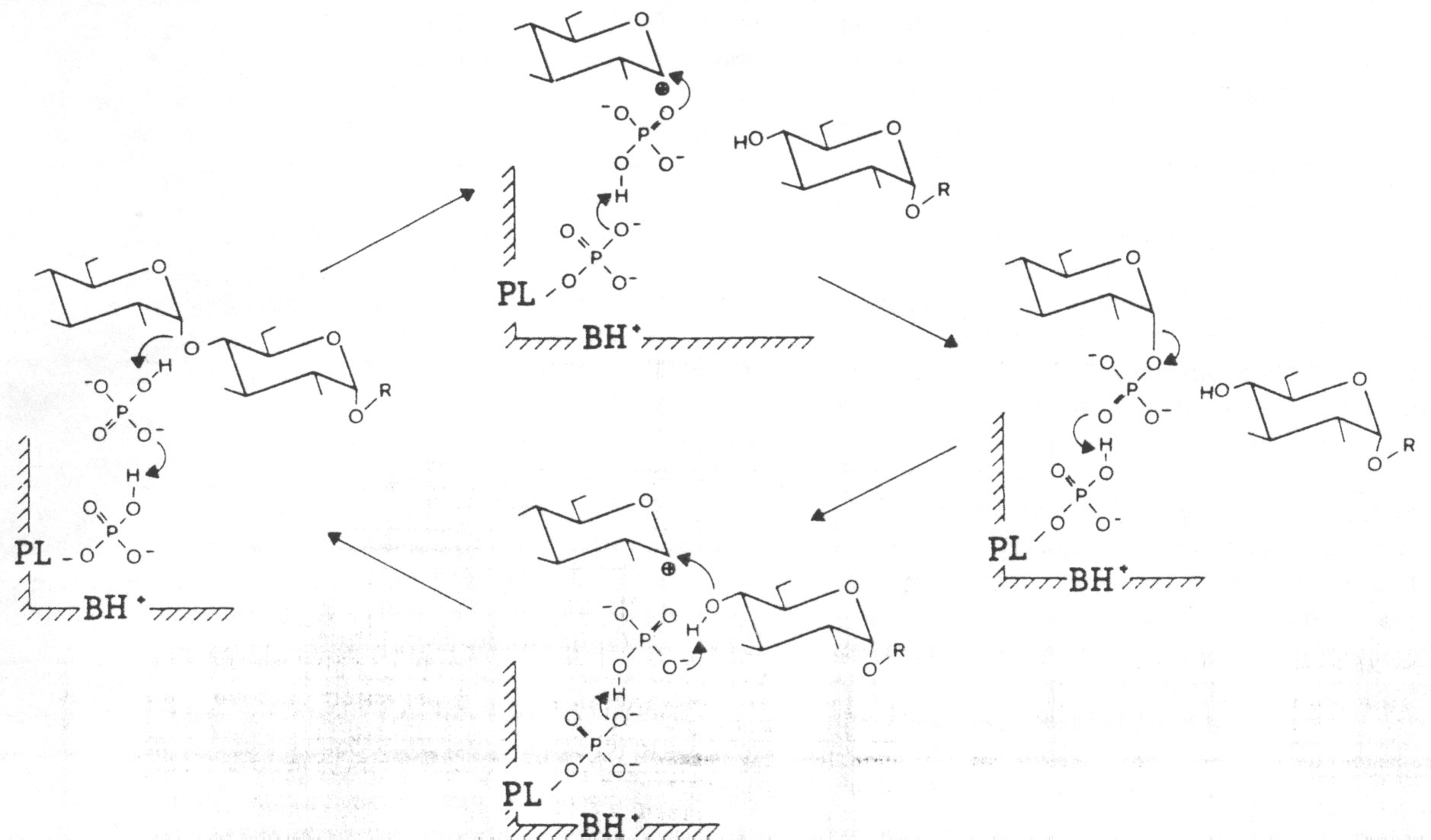
In the first step (left side), the substrates glycogen and phosphate have enterted the active site. The glycosidic bond is cleaved when the electrons from one side of the glycosidic monomer donate electrons to the substrate phosphate group. This leaves the glycogen monomer hydorxylated at the 4' OH position and leaves the 1' position of the glucose monomer with a positive charge. In this step, PLP acts as a hydrogen shuttle, it gives up its phosphate group to the substrate phosphate group.
In the next step (top), the substrate phosphate donates electrons to the carbocation at the 1' position on the glucose monomer, forming G-1-P and releasing it as a produuct. The PLP group takes back the proton it donated to the substrate phosphate group. Thus the cleavage step is simply a concerted two-step reaction.
The next two steps in the diagram show essentially the reverse reaction of the first two steps. Again this would occur under conditions of low phosphate concentration, and high G-1-P concentration. (Modified from Palm et al 1104).