Biogenesis of TPQ
Biogenesis of TPQ

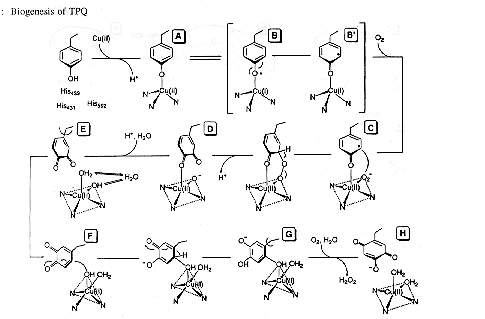
Biogenesis of 2,4,5-trihydroxyphenylalanine quinone (TPQ) from a post-translational modification of tyrosine 405 in the copper amine oxidase. This process is autocatalytic in the presence of oxygen, meaning no other cofactors or enzymes are needed for transformation for this protein bound tyrosine to TPQ. In the first step, the copper II ion binds to the apoprotein to form the Cu(II) complex A. Complex A is in equilibrium with a Cu(I)-tyrosyl radical B. The unpaired spin in B is delocalized over the aromatic ring which activates the ring carbon atoms B'. The Cu(I) complex reacts with dioxygen to form an activated oxygen complex C, shown here as a Cu(II)-superoxide. This activated oxygen complex attacks the tyrosyl radical forming a complex D, a dopaquinone bound to a Cu(II) oxide/hydroxide/aqua complex. This Cu(II) oxide/hydroxide/aqua species should exchange with water, permitting the oxygen incorporation into TPQ, E to F. Rotation of the dopaquinone aromatic ring brings C2 close to the equatorial water/hydroxo ligand F. Nucleophilic attack on the dopaquinone by Cu(II)-OH- results in the topa G. In the presence of dioxygen, topa is oxidized resulting in TPQ. After the TPQ dissociates, the Cu(II) atom binds a water molecule and returns to a square-pyramidal geometry H. 1
Figure taken from Matthew C. J. Wilce, et.al.