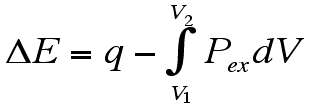Anti-thermophobic chemistry
Before looking at proteins we shall turn our attention to the basic structure of common biochemical motiffs that function as antifreeze molecules.
Competent biophysicalchemists are well versed in the lovely polyethyleneglycol (PEG).


Although we will spend most of our time looking at proteins from hyperthermophiles, a quick look at Hplc-12 Type III Antifreeze Protein From Ocean Pout Macrozoarces Americanus should satisfy our needs to warm any cold fish out there.
spin away!
|
||||||||||
But we don't find organisms making our windshield washer solution. Fortunately the most common antifreeze biostructure is only slightly more complicated chemically.
The generic form for an antifreeze protein consists of a polyamino backbone branched with a sugarish molecule. More specifically, the polymer has the chemical formula:
Beta-galactosyl-(1-->3)-alpha-N-acetylgalactosamine in glycosidic linkage to the tripeptide
tripeptide: [alanine-alanine-threonine]n , where n = 4,5,6,12,17,28,35,45, or 50
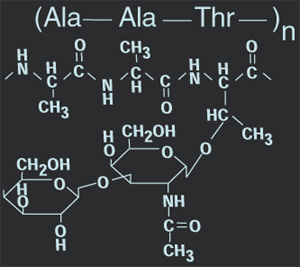
Considering the extent to which these proteins have been studied it may come as a surprise that there is no consensus mechanism by which it acts. Not unlike some futuristic sorbet the antifreeze molecule binds to ice crystals and turns them into a softer more manageable slush. But we are investigating proteins that are in love with heat, not just against freezing. The transition from a primary or secondary polypeptide to higher levels of structure is characterized by the OB fold in sac7d and Sso complexes. As the free energy of folding reaches a mininum, the intrinsic vibrational energy of the protein slows and concomitantly we may observe the protein acting as a unit to seek heat.
Ma^hematically this can be expresssed as a differential equation
dq=C(Tf-Ti)
In classical thermodynamics, the heat capacity IS, of course, held constant within fixed system boundaries. However, as we probe the protein at the molecular level, a quantum mechanicall or semi-empirical molecular orbital modeling approach needs to be considered. So logic follows that the properties of the protein can be expressed using classical thermodynamics when the entire protein is set to behave as an unit, but if we wish to understand how the space within the space constraints of the protein is occupied by matter, we must change our approach.
Thus I will provide a framework for determining what the differential volume component to the following integral means in a biophysical chemical sense