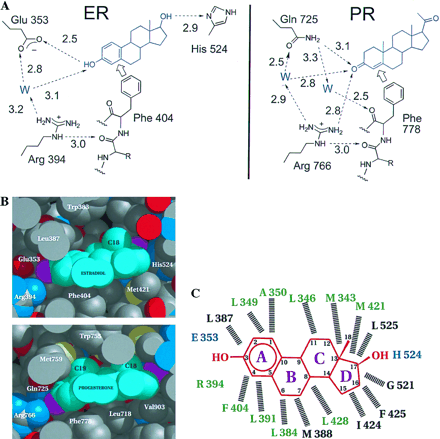
Estrogen Binding
Here can be seen the entire ER from the human genome. The estrogen moleculesPhe404
An alternative picture of the binding pocket can be seen below.

The pictures relating to PR and progesterone are from another study relating the progesterone receptor and the ER.
The binding pocket of estrogen can be seen as highly hydrophobic, with the residues of Glu353 and His524 containing some of the sole polar residues in the area.
The binding of estrogen allows monomers of the ER to homodimerize, and allows the 2 DNA-binding domains to cooperatively bind to the DNA.