 |
|
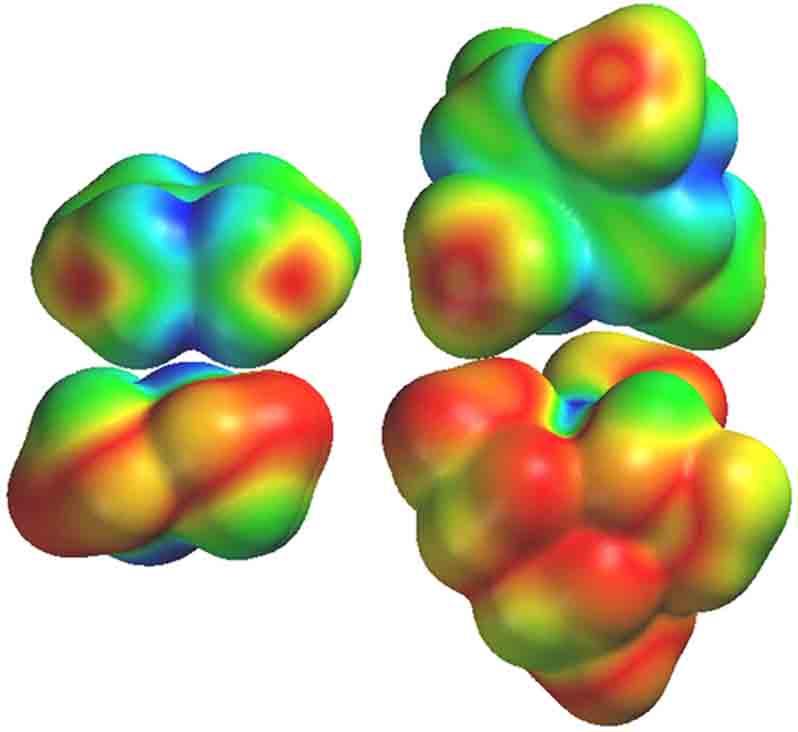
Figure Caption: HOMO electron density function mapped on the surface density function for Me2N)20 (lower left) and the LUMO mapped on the surface density function of Me2N)2+ (upper left). Blue indicates the region of highest density for the mapped function; red is zero density. The equivalent functions are displayed for the Et2N)20– Et2N)2+ reactant pair on the right. Geometry optimization and electron density function maps computed at the DFT B3LYP/6-311G* level using Spartan 02.
Metalloprotein Electron Transfer
The metalloprotein work has focused on the role of stereochemistry in the selectivity displayed by a metallprotein in its electron transfer reactions with coordination compounds. Our research has observed stereoselective electron transfer with three different proteins: horse cytochrome c, spinach plastocyanin, and human copper-zinc superoxide dismutase. For each protein studied, we have been able to assign the likely site of substrate binding prior to electron transfer and this has greatly enhanced our ability to interpret the observed stereoselectivity. The results on cytochrome c and plastocyanin have been published in J. Am. Chem. Soc. and Inorganic Chemistry and the superoxide dismutase study has been published in a special volume of Inorganica Chimica Acta devoted to electron transfer. This work has involved more than 40 undergraduate co-workers.
UW-Eau Claire Undergraduates
Most of the work described here has been done by UW-Eau Claire undergraduates. Nearly 60 undergraduates have been involved in my research to date and 75% of them have gone on to receive advanced degrees or have advanced degree work in progress. Many of have been coauthors on the papers listed in recent publications and have presented papers at national meetings of the American Chemical Society (ACS). For example, on the Accounts of Chemical Research paper described above 15 students over a ca. 12 year period contributed to the work and most of them have gone on to do advanced work in the chemical sciences (Craig Stolen, Ph.D. in Biochemistry UW-Madison; Molly Accola, Ph. D. Immunology-Pathology Harvard University; Yvonne Gindt, Ph.D. UC-Berkeley Biophysics; Steven Gullerud, Ph.D. Materials Science Stanford University; Troy Seehafer, chemical industry; Jobiah Sabelko, Ph.D. Chemistry University of Illinois; Jennifer Brandt, M.S. Chemistry University of Minnesota; Qinling Qu, M.S. Chemical Engineering Purdue University; Xi Chem, Ph. D. Biochemistry University of Rochester; Amy Odegard, Ph.D. in progress University of Wisconsin Madison and Harvard University; DeWayne Halfen, Ph.D. in progress University of Arizona; Katy Splan, Ph.D. in progress Northwestern University; Jamie Gengler accepted to Ph.D. program in Chemistry at Arizona State University; Teresa Jentzsch accepted to Ph.D. program in Chemistry at UC-Berkeley; Jessica O'Konec accepted to Ph.D. program in Pharmacology at University of Wisconsin-Madison). !2 of these students were coauthors of papers cited in the Accounts paper and two were coauthors on unrelated work.
Most recently Teresa Jentzsch (right) and Jessica O'Konec presented a paper at the National Meeting of the ACS in the spring of 2001. The picture below is from that presentation. Teresa and Jessica are also coauthors on our 2001 paper in J. Chem. Soc. Perkin 2.

![[UWEC Web]](../Media/smuwec.gif) [Colleges] [Chemistry Dept.] [Chemistry Faculty] [Professional Page]
[Colleges] [Chemistry Dept.] [Chemistry Faculty] [Professional Page]