Aramid Fibers
Introduction
In the research labs at E. I. Du
Pont de Nemours & Company, Inc., in 1965 two research
scientists, Stephanie Kwolek and Herbert Blades, were working in a corporate lab
to create a new fiber. The technology they developed had enhanced strength, was
lightweight and very flexible. The new
fiber, called Kevlar, could be offered in many different forms. One of the most popular uses of Kevlar came
in the form of bullet-resistant vests that police officers have relied on for
over 25 years. The greatest attribute of
the fiber was strength it provided in a very lightweight form, that was both
comfortable and gave a wide range of movement to the officer. This discovery came from a very chemically
similar compound called Nomex. The
creation of this fiber gave birth to thermal technology, which combined heat
and flame resistant properties along with advanced textile characteristics.
The production of aramid fibers
known under their trademark names Kevlar® and Nomex.® have unique and
beneficial properties. These two aramids
are similar in basic structure and are sometimes produced in the same
production plants. The difference is in
their structure, Kevlar® is a para-aramid while Nomex® is a meta-aramid. An aramid is a polyamide where at least 85%
of the amide bonds are attached to aromatic rings. The first aramid produced was called Nomex®
introduced by Du Pont in 1961. For this
report we will dissect each fiber separately.
Kevlar®
History
Kevlar® was originally developed in
the 1960’s with the chemical name of poly-paraphenylene terephthalamide; but
chemists to this day still do not understand why the fiber is so strong. First introduced commercially by Du Pont in
1972, the fiber has similar competitors in Twaron and Technora. Kevlar was originally developed as tire chord
material for belts and carcasses in radial tires. The common uses for Kevlar®
today include: adhesives and sealants,
ballistics and defense, belts and hoses, composites, fiber optic and
Electro-mechanical cables, friction products and gaskets, protective apparel,
tires, and ropes and cables. These
include items such as trampolines and tennis rackets.
Characteristics
The
resounding characteristic of Kevlar is its remarkable strength. This very strong fiber has made its biggest
impact in the ballistics defense where it’s used in bulletproof vests. It is stronger than fiberglass and five times
stronger than steel on a pound-for-pound comparison. The high tensile strength and modulus are
characteristics of all the Kevlar fibers, with Kevlar 49 and Kevlar 149 showing
an even higher modulus. Kevlar’s chains
are ordered in long parallel chains, and the key structural feat is the benzene
aromatic ring that has a radial orientation that gives the molecule a symmetric
and highly ordered structure that forms rod-like structures with a simple
repeating backbone. This creates an
extremely strong structure that has few weak points and flaws. The table provided below shows the various
characteristics of Kevlar fibers and where compiled from both the Chemical
Economics Handbook and Encyclopedia of Chemical Technology, Vol. 19.
|
Properties of Commercial Aramid Fibers |
|
|||
|
|
|
|
|
|
|
Fiber type |
Density, (g/cm3) |
%Elongation |
Modulus, Gpa |
Tenacity |
|
Kevlar 29 |
1.43 |
3.6 |
70 |
20-23 |
|
Kevlar 49 |
1.45 |
2.8 |
135 |
20-26 |
|
Kevlar 119 |
1.44 |
4.4 |
55 |
N/a |
|
Kevlar 129 |
1.45 |
3.3 |
99 |
N/a |
|
Kevlar 149 |
1.47 |
1.5 |
143 |
18 |
|
Nomex |
1.38 |
22 |
17 |
5.8 |
Notice the much higher modulus and lower %
elongation from Kevlar 49 and 149.
All of the general features of Kevlar listed
here are taken from Du Pont’s web homepage:
·
·
High Tensile Strength
at Low Weight
·
·
Low Elongation to Break
·
·
High Modulus
(Structural Rigidity)
·
·
Low Electrical
Conductivity
·
·
High Chemical
Resistance
·
·
Low Thermal Shrinkage
·
·
High Toughness
(Work-To-Break)
·
·
Excellent Dimensional
Stability
·
·
High Cut Resistance
·
·
Flame Resistant,
Self-Extinguishing
These features give a good picture on why
Kevlar is a popular choice for all protection and casing purposes; low
conductivity and self-extinguishing, flame resisting characteristics have made
it a component for wire casing and fire fighting protection. The interesting thing is that it has a high
elongation at break at around 4%, however it is commonly used in fiber that
includes Lycra spandex.
Chemistry/Manufacture
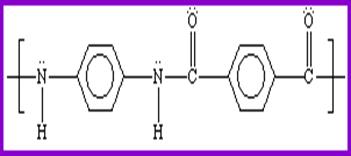
KEVLAR® is a crystalline molecule
that consists of long molecular chains that are highly oriented and show strong
intermolecular chain bonding in the para position. It is made from the reaction of
para-phenylenediamine (PPD) and molten terephthaloyl chloride. The production of p-phenylenediamine is
difficult because of the diazotization and coupling of aniline. The reaction compounds involving the
production Kevlar using p-phenylenediamine and terephthaloyl chloride is shown
below.
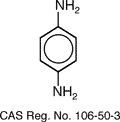
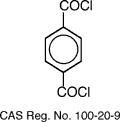
style='mso-tab-count:2'>
The PPD and the terephthaloyl chloride are
reacted by using N-methylpyrrolidone as a reaction solvent. The structure for
poly-paraphenylene terephthalamide is shown below.
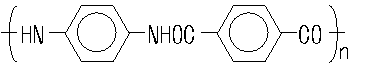
The resulting polymer is filtered,
washed and dissolved in concentrated sulfuric acid and is extruded through
spinnerets. It then passes through a
narrow duct and goes through the wet spin process where it is coagulated in
sulfuric acid. The filament can take two
different paths at this point. It can be
formed into a yarn, washed and dried which is wound into spools that produces a
modulus of 400-500 g/denier. Conversely, the filament can go under further heat
treatment with tension and produce a fiber with a modulus of 900-1000
g/denier. The end product can take
several forms. It can form filament
yarns, pulp, or spun-laced sheets and papers.
Economic Impact
The production of fibers like Kevlar
is really an oligopoly. Du Pont, being
the producer of Kevlar is the largest producer para-aramids in the world. Du Pont currently produces in three
countries: the United States, Northern Ireland, and Japan. These three sites have a production capacity
of 65.9 million pounds of the 94.7 million pounds of total aramid fibers
capacity. The other producers are Aramid
Products in the Netherlands, which makes Twaron and Teijin Ltd of Japan, who
makes Technora. Russia also produces a
very low percentage of para-aramids called Fenylene.
Below is a production table for all para fibers
in the last two decades. As of 1998,
Kevlar accounted for 85% of the global market of para-aramid fibers. Production in Western Europe and Japan has
jumped up greatly in the last ten years.
All of the production in the United States is done by Du Pont to produce
Kevlar. Also Du Pont accounts for about
one-third of the total production in Europe and about one-half of the
production in Japan.
|
World Production of Para Fibers (millions of pounds) |
|||||
|
|
|
|
|
|
|
|
|
United States |
Western Europe |
Japan |
Russia |
Total |
|
1979 |
13 |
0 |
0 |
<1 |
13 |
|
1986 |
29 |
<1 |
0 |
2 |
31 |
|
1988 |
29 |
6 |
<1 |
2 |
37 |
|
1990 |
29 |
10 |
1 |
3 |
43 |
|
1991 |
26 |
10 |
4 |
2 |
42 |
|
1992 |
23 |
11 |
7 |
2 |
43 |
|
1993 |
23 |
12 |
7 |
2 |
44 |
|
1998 |
31 |
16 |
8 |
3 |
58 |
***Figure from this table taken from the Chemical
Economics Handbook
Consumption of para-aramids in the three major
regions: United States, Western Europe, and Japan hit 39 million pounds in 1993
and increased to 47 million pounds in 1998.
The growth of Kevlar has not yet met it’s full
potential. The rapidly growing uses for
Kevlar include ballistic protection in Western Europe, truck and bike tires,
and with it’s lightweight dielectric properties, tension reinforcement for
fiber optic above ground cables and protective coverings for underground and
underwater fiber optic cable. Of all the Kevlar imported; 50% is used for tire
manufacture, while the rest is used for fiber optics, brake materials, and for
industrial fabrics. Dunlop Tire Corp.
has begun to make a tire that is 30% lighter than traditional tires and that
eliminates the steel belt and bead wire.
The only catch that’s holding back a full scale use of Kevlar is its
price; 1,500 denier is commonly used for tire cord, hoses and belts costs $12.00
per pound, while the other common grades of Kevlar range in the $13.00 to
$15.00 range. Outside of the U.S., the
same 1,500 denier fiber costs $23.00-27.00 per pound. Even with the expanding market as it
currently is, widespread growth will not be realized until the costs of
production falls.
Nomex®
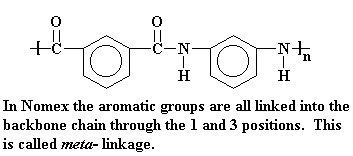
History
NOMEX® was developed by DuPont for
in 1961 for products that needed dimensional stability and good heat
resistance. Nomex® products are used in
protective apparel, hot gas filtration, and automotive hoses, electrical
insulation, aircraft parts, and sporting goods.
Characteristics
The properties of Nomex include
great electrical insulation properties at high temperatures. Nomex does not flow or melt upon heating and
doesn’t degrade or char at temperatures until well over 370 degrees
Celsius. The compound that is usually
found in fire-fighters coats and airline seat covers is Nomex III, which is a
composite of 95% Nomex and 5% Kevlar.
The Kevlar adds stability and tear resistance to the material. The general properties of Nomex are listed
below.
·
·
Heat and Flame
Resistant
·
·
High Ultraviolet
Resistance
·
·
High Chemical
Resistance
·
·
Low Thermal Shrinkage
·
·
Formable for Molded
Parts
·
·
Low Elongation to Break
·
·
Low Electrical
Conductivity
This properties cause paper made by Nomex to be
stronger and tougher than regular cellulosic papers. Overall, Nomex® is both thermally and chemically
very stable. The difference between
Kevlar and Nomex is the location of the amide linkages on the aromatic
ring. Those differences cause Nomex to a
lower modulus and tensile strength and a higher elongation and solubility in
organic solvents.
Chemistry/Manufacture
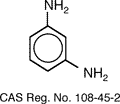
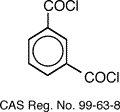
Nomex®, is a meta-aramid fiber
created by Du Pont in 1961. The chemical
name of Nomex is poly (m-phenylene isophthalamide), which is produced from the
reaction of m-phenylenediamine and isophthaloyl chloride whose structures are
shown below.
The solution is dry spun through
spinnerets. The remaining solvent is
evaporated, the filament is washed and wound into tow, heated, and finally
stretching into rolls at a temperature of 150 degree’s Celsius. Nomex can be produced as a continuous
filament yarn, staple, spun yarn, floc, pressboard, paper, needle felt, or as a
fabric. Next we will take a look at the
economics of producing Nomex.
Economic Impact
The growth of meta-aramid fibers has
grown steadily over the last 10 years. At the same time the U.S. share of production
has fallen 19% from 1990 to 1998 from 81% to 62%. This drop is largely due to the growth of
production in Western Europe, from no production in 1990 to 21% of the market
share in 1998. The table below shows
production patterns of meta-aramids since 1979.
|
World production of Meta-Aramid fibers (millions of pounds) |
||||||
|
|
|
|
|
|
|
|
|
|
|
United States |
Western Europe |
Japan |
Russia |
Total |
|
1979 |
|
12 |
0 |
<1 |
<1 |
12 |
|
1986 |
|
18 |
0 |
2 |
1 |
21 |
|
1988 |
|
20 |
0 |
2 |
2 |
24 |
|
1990 |
|
21 |
0 |
4 |
2 |
26 |
|
1991 |
|
23 |
0 |
4 |
1 |
28 |
|
1992 |
|
24 |
0 |
4 |
neg |
28 |
|
1993 |
|
26 |
2 |
4 |
neg |
32 |
|
1998 |
|
26 |
9 |
5 |
2 |
42 |
***Figures taken from the Chemical Economics Handbook
The world production has more than tripled in
the last three decades while consumption in the U.S. only grew 60%. This is due the great increase of consumption
in Western Europe and growth in Japan.
The uses of this consumption is largely for the production of paper
electrical uses, as insulators in dry transformers, motors, and transformers
which account for 49% of all U.S. consumption.
In the textile industry, fire resistant fabric accounts for 19% and
filtration 17% of all U.S. consumption.
Overall, the expected annual growth rate for meta-aramids is suppose to
average 3% a year until 2003. The
textile industry is responsible for the production of fire-resistant clothing
and seat covering in airline seats. It
also has established a market in asbestos replacement, thermal insulation and
as a fiber that prevent static electricity buildup. The prices for meta-aramid fibers range
greatly. The staple 1.5-denier fiber cost $11.50 per pound while continuous
filament yarn of 200 denier cost $25.00 per pound. Even more, 1,200 denier filament yarn costs
39.00 per pound!
Summary
In this paper, I have dissected the
chemistry and the growing markets of the specialty fibers Kevlar and
Nomex. Each of these fibers has shown
extensive growth over the last few decades with growth expected to continue
over the next several years. This poses
the question on whether we should expand into these markets and capitalize on
this growth or sit by the wayside. In my
opinion, the outlook for polyamids such as Kevlar and Nomex aramids is very
good. Du Pont, an established company
whose products are well known and trusted, dominates the production of these
fibers. Over the next several years, Du
Pont is going to profit from the production of these fibers. With the established name brand and quality
that Du Pont already holds, the barriers to enter the market are too great for
any company to start up and take their strangle hold over the aramid
market. The invention of these fibers
grew from the research from making very basic items into one of the most
structurally sound products made today.
Bibliography
“Aramids.” About.com.
1996. <http://composite.about.com/industry/composite/gi/dynamic/offsite.htm?site=http%3A%2F%2Fwww.psrc.usm.edu%2Fmacrog%2Faramid.htm> (15 Nov. 2000)
Chang,
Alen: Hung, Richard; Lew, Katherine, Function and Performance of Kevlar, pdf
file, http://www.mse.berkeley.edu/classes/matsci102/Kevlar.pdf
Du
Pont Website:
“Kevlar” <http://www.dupont.com/afs/kfeatures.htm> (15 Nov. 2000).
Du Pont Website: “Nomex” < http://www.dupont.com/nomex/> (15 Nov. 2000).
“Flame
Retardants.” Ullmann’s Encyclopedia of Industrial Chemistry. 1988. Vol
11.
GE website:
http://www.complas.com/kevlar.asp
Groce,
Donald F. “Cotton, Nylon, Lycra Spandex and Allergies.” Latex Allergy News. Sept.
1996. <http://latexallergylinks.tripod.com/lycra.html> (15 Nov. 2000)
“Polyamides
(General)” Encyclopedia of Chemical Technology. 1996 ed. Vol. 19.
p.506-508, 519-523.
Reisch,
Marc. “What’s that Stuff?” Chemical and Engineering News. 15 Feb 1999
<http://pubs.acs.org/cen/whatstuff/stuff/7707scitek4.html> (15 Nov. 2000)
“Spandex
Fiber (Elastane).” Fibersource. <http://www.fibersource.com/f-tutor/spandex.htm>
(November 2000).
“Specialty
Organic Fibers” Chemical Economics Handbook. 1999 ed. 542.7003,
542.7000.
University
of Missouri-Rolla website: http://www.umr.edu/~wlf/Synthesis/kevlar.html
Last
Updated: 30 April 2001