Kyle Caspary
Chem 115 Research Project
Nuclear Fusion –
Keeping the Universe Alive
Since the Beginning
How do the stars shine? How is it that our sun emits enough energy to sustain the lives of billions of people as well as the lives of countless other life forms? The answer to both of these questions is nuclear fusion. The process involving two small nuclei combining together to form a larger nucleus as well as energy is known as nuclear fusion. This type of reaction is prevalent in stars and more importantly to Earth, our sun. Many stars have been undergoing nuclear fusion reactions for extremely long times. Imagine the possibilities if we could somehow harness that almost everlasting source of energy. It is not as implausible as it may seem. Even now, more and more research is being done to make this prospect a reality.
There are a couple ways to look at how the fusion reaction produces energy. The first utilizes one of Einstein’s most well known equation, E = mc2, where E represents energy, m stands for mass and c is the speed of light. What this equation gives is a conversion of mass to energy. If you take the sum of the masses of the particles going into the fusion reaction and compare them to the sum of the masses of the particles that are formed after the fusion reaction, you discover that there is a change. For the fusion reactions that are useful for energy production, the final mass of the particles is lower. E = mc2 shows us that there is energy released. The amount of energy released depends upon the specifics of the reaction. Energy must be absorbed for fusion reactions that produce elements heavier than iron. The other way to look at it is in terms of binding energies. The binding energy of a system is the amount of energy that is needed to separate the system into all of its individual components. If the formed nucleus of a fusion reaction has a larger binding energy than the reactants then the reaction is exothermic.
Nuclear fusion is very hard to achieve. This is because it requires very rare conditions before it can proceed. Just about all natural nuclear fusion occurs in stars. In the stars, hydrogen is converted into helium. After all the reserves of hydrogen begin to diminish, one of two things can occur. The first case is that the star will stop burning. The second will only occur if the star is large enough. In this case, the star will start utilizing helium as a new fuel and it will continue its fusion process to make larger elements. This process will continue until the reaction begins to produce iron. All of the fusion reactions that occur to produce elements larger than iron no longer release energy. So, after this point, the star will begin to run out of fuel.
Although there are
many different fusion reactions, the ones that are most likely to succeed
involve the use of smaller nuclei. A
smaller nucleus has less charge and therefore the nuclei involved in the
reaction will encounter a smaller repulsive force due to their like
charges. In order for these two nuclei
to react, they must collide with enough energy so that they get close enough
for the strong nuclear forces to fuse them together. In order to do this, the nuclei must have enough kinetic energy
to overcome the repulsive forces due to their like charges. This leads to one of the drawbacks of fusion.
It requires extremely high temperatures in order for the kinetic energies of
the reactants to be this large. The
reaction that is most likely to be used for energy production is the reaction 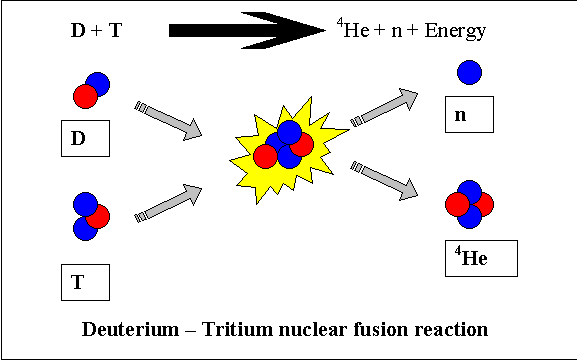 that
fuses two isotopes of hydrogen, deuterium and tritium. This is not the same reaction that occurs in
stars. In the stellar fusion reaction,
hydrogen atoms are fused into helium atoms.
The extra neutrons in the isotopes of hydrogen make it easier for them
to get close enough to fuse together. For
the deuterium – tritium reaction, the temperature required is over 100 million
Co. At this temperature, the
fuel has become a plasma or an ionized gas.
Another reason this reaction is favorable is the overall abundance of
the reactants. Deuterium can be found,
in low, but attainable amounts in seawater.
Tritium can be formed from lithium, which is also relatively abundant in
the earth.
that
fuses two isotopes of hydrogen, deuterium and tritium. This is not the same reaction that occurs in
stars. In the stellar fusion reaction,
hydrogen atoms are fused into helium atoms.
The extra neutrons in the isotopes of hydrogen make it easier for them
to get close enough to fuse together. For
the deuterium – tritium reaction, the temperature required is over 100 million
Co. At this temperature, the
fuel has become a plasma or an ionized gas.
Another reason this reaction is favorable is the overall abundance of
the reactants. Deuterium can be found,
in low, but attainable amounts in seawater.
Tritium can be formed from lithium, which is also relatively abundant in
the earth.
How do we go about containing 100 million Co plasma? There are three methods of doing this. The first method of containment is rather simple. First, you obtain an amount of reactants about 1/10 the mass of the sun. Then, you let gravitational forces compress the reactants until the pressure and temperature inside the mass is large enough to start the fusion reaction. This works very will for stars in outer space, however, since this amount of mass would be incredibly large, this is not an option for energy production on earth. In fact, plasma containment on earth is not very simple at all.
 The two remaining containment
methods are magnetic confinement and inertial confinement. In magnetic confinement, magnetic fields are
used in order to prevent the ionized units in the plasma from escaping and to
also prevent them from touching the walls of the container and cooling. The magnetic fields cause the charged
particles to change direction and move so that they remain contained. Depending on the shape of the magnetic
field, the resultant motion may vary. It
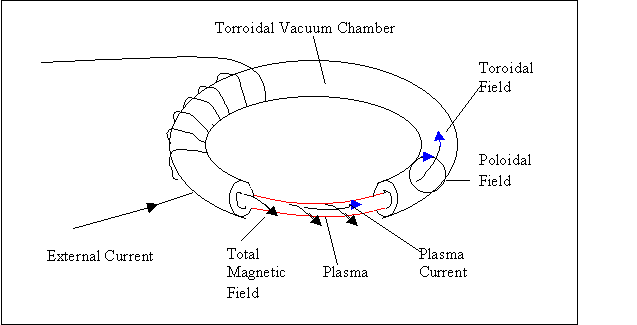
turns out the most effective shape for plasma confinement is a toroidal, or
doughnut shaped. A very complicated
device known as a tokamak was first developed in the USSR. It utilizes the toroid shape as well as
using complicated magnetic fields. One
field is produced along the axis of the torroid itself. This is the torroidal field. The second, sending a current through the
plasma itself creates a polloidal field.
When these fields combine, they produce a spiraling field through the
toroid.
The two remaining containment
methods are magnetic confinement and inertial confinement. In magnetic confinement, magnetic fields are
used in order to prevent the ionized units in the plasma from escaping and to
also prevent them from touching the walls of the container and cooling. The magnetic fields cause the charged
particles to change direction and move so that they remain contained. Depending on the shape of the magnetic
field, the resultant motion may vary. It
turns out the most effective shape for plasma confinement is a toroidal, or
doughnut shaped. A very complicated
device known as a tokamak was first developed in the USSR. It utilizes the toroid shape as well as
using complicated magnetic fields. One
field is produced along the axis of the torroid itself. This is the torroidal field. The second, sending a current through the
plasma itself creates a polloidal field.
When these fields combine, they produce a spiraling field through the
toroid.
Inertial confinement is the third method for containing a plasma. In inertial confinement, a small piece of deuterium – Tritium ice is the target of very intense laser beams. The ice is struck from several directions at the same time. The result of this impact is a increase in density. The density of the pellet is increased to greater than the density of the sun and the fusion reaction occurs. A major problem with this method is the inability of the lasers to hit the ice exactly symmetrically. No matter what method is used, they are all trying to obtain the same thing. They are all attempting to reach a value known as the Lawson criterion. In 1957, J. D. Lawson showed that the condition for achieving ignition, a self sustaining reaction that continues without external heating, depends upon the ion density n and the confinement time t such that:
![]()
Currently there are many countries that are taking part in fusion research. The leaders include the European Union, USA, Russia and Japan. Previously, fusion research had ties to atomic weapons development. It was declassified because of the 1958 Atoms for Peace conference in Geneva. After a big breakthrough at the Soviet tokamak, fusion research became very large. This was around the 1970’s. The increased cost and the ever-increasing complexity of the devices caused a large amount of international collaboration. It was the only way to proceed forward. In 1978, the JET project was launched in the UK. In 1983, JET produced its first plasma in 1983. By 1991, it was seeing promising experiments utilizing deuterium – tritium reactions. As the research pushes forward, so will the results, until a final goal is realized.
Fusion power could mean a great deal. It could provide an abundance of energy as well as decrease the overall impact on the environment because it produces no atmospheric pollution. Unlike nuclear fission, there would be no risk of a malfunction in a fusion reactor. However, this is not to say that there are no dangers at all associated with fusion. The expelled high-energy neutrons could cause some of the components to become radioactive. The volume of such waste would be comparable to that of a fission reactor, but the radioactivity of this waste would be relatively short lived in comparison. These aren’t the only concerns with respect to fusion. The American Association for the Advancement of Science (AAAS) raised many concerns in 1973. One hazard could arise from an accident involving the magnetic system. The energy required to provide the magnetic field would be similar to the energy of a lightning bolt. A lithium fire could also be a possible concern. Lithium is very reactive and could release a lot of energy if something went wrong. The AAAS’s biggest concern involved the release of tritium into the environment. Tritium has the ability to penetrate concrete, rubber and even some steel which would make containing the radioactive substance rather difficult. It could also incorporate itself with water and make some of the water radioactive. Also, while it does have a relatively short half life, 12.4 years, it is still long enough that contaminated water could remain toxic for as much as 100 years after it is created, no matter what the form. It is estimated that each fusion reactor could release some amount of tritium through leaks. This is one reason why long term hopes for fusion do not include tritium and look for a possible deuterium – deuterium fusion process.
Despite some possible dangers and its complexity, fusion could prove to be a necessary and useful energy in the future. With many advantages over fossil fuels and nuclear fission, nuclear fusion seems to be very appealing. With research advancing as it is, who knows what may lie just around the corner with this innovative process.
Bibliography
1.
Atkins & Jones (1999). Chemistry:
Molecules, Matter, and Change. New
York: W.H. Freeman and Company.
2.
Giancoli (2000). Physics for
Scientists & Engineers with Modern Physics. Upper Saddle River: Prentice Hall.
3. “Nuclear
Fusion Basics”
4. New
Mexico Tech “Fusion: An Alternative Energy Source “
http://www.physics.nmt.edu/~plasma/Fusion/index.html
5. World
Nuclear Association “Nuclear Fusion
Power”
http://www.world-nuclear.org/info/inf66.htm
6. Robert
F. Heeter Answers to Frequently Asked
Questions about Fusion Research
http://fusedweb.pppl.gov/FAQ/fusion-faq.html