Radiocarbon Dating: History and Process
Jessica Berti
Radiocarbon dating is one of the most significant scientific discoveries of the twentieth century. With radiocarbon dating archeologists and other scientists no longer rely solely on relative ages and can spend time researching how and why things happened rather than when.
Radiocarbon dating was developed in the late 1940's by a team of scientists at the University of Chicago who were lead by Professor Willard F. Libby. For his work, Libby received the Nobel Prize in Chemistry in 1960. Today over one hundred thirty laboratories around the world date samples sent into them. One Google search yields many labs advertising their reliability and speed.
The radiocarbon, or C14, method is applied in many scientific fields such as hydrology, oceanography, archeology, and geology. Many famous things have been radiocarbon dated such as the Dead Sea Scrolls and the Shroud of Turin. With radiocarbon dating scientists are able to discover more about these artifacts because they know how old they were.
Background Information
Radiocarbon dating is reliant on a carbon isotope, one of two or more atoms having the same atomic number but different atomic masses (1), known as carbon 14. There are three naturally occurring isotopes of carbon. They are C12, C13, and C14. Carbon 14 is extremely rare: one carbon 14 atom exists for every 1,000,000,000,000 carbon 12 atoms in living matter. (2) Carbon 14 is formed in the upper atmosphere when cosmic rays interact with atoms causing them to loose neutrons. These neutrons batter the nitrogen causing it to loose a proton. This is now called carbon 14.
14N + n -> 14C + p
(n represents a neutron from the interaction of a cosmic ray with another atom and p represents the proton lost)
From the 14C 14CO2 is formed. It enters the carbon cycle of the earth in plants through photosynthesis, animals who eat those plants, and up the food chain. The 14C cycle continues. The amount of 14C in an organism stays at approximate equilibrium throughout the life of the organism because it is being replenished through photosynthesis or the consumption of plants and other animals. This ratio is approximately 1/1012 of carbon 14 atoms to carbon 12 atoms in living things. (1) This is assumed to be the same as the atmosphere. When an organism dies there is no longer an uptake of C14 from the environment, only decay. However, the amount of carbon-12 stays constant. The radioactive C14 atoms decay with a constant half-life Libby calculated at 5568+- 30 years. (2) A half-life is the time needed for half the initial number of radioactive nuclei to disintegrate. (1) At approximately 50-60,000 years ten half-lives have past and the amount of 14C in the sample is so small that radiocarbon dating is no longer possible. Therefore, other methods such as OSL (Optically Stimulated Luminescence dating) or isotopes with longer half-lives can be used. (2)
When C14 decays a weak beta particle, or electron, is released. One beta particle has 160 keV of average energy. This energy is used in spectrometry to detect the amount of C14 present. C14 converts back to 14N in the following fashion.
14C -> 14N + b
(b represents the beta particle)
Almost any once living thing consisting of carbon can be dated. Some examples include charcoal, bone, shells, leather, and even paper. These samples are dated by comparing the ratio of 14C atoms to 12C atoms. Things that were non-living such as rocks or extremely old carbon-based compounds such as fossil fuels cannot be dated.
In the late 1950's and early 1960's researchers studying US bristle cone pine and German and Irish oaks discovered fluctuations in the C14 concentration. Measurements of the Libby half-life showed that Libby was about .3% too low. The Cambridge half-life that was found from this research is 5730+- 40 years.
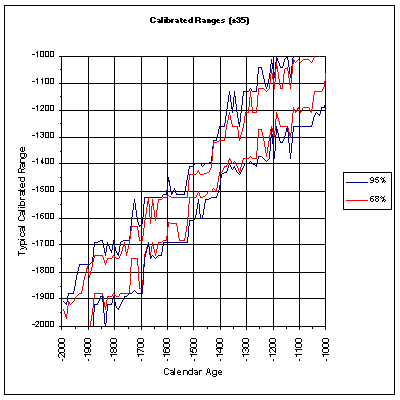
We cannot be certain of the exact year in which a sample came from: only make and educated estimation. Research of tree rings has revealed that the amount of radiocarbon has not been constant. In the 1950’s and 60’s many nuclear bombs were blown up causing a rise in the amount of radiocarbon in the atmosphere. Because of this dating a relatively young sample is very difficult. This has been a trade off, however, because scientists were able to track radiocarbon through the environment and learn about the transportation of radiocarbon. (2) However, with calibration we can determine a range in which we are 95% certain that a sample originated in. Here is a table of calibrated ranges for samples from 2000 to 1000 BC. As you can see, the range is quite substantial for some calendar years.
http://www.rlaha.ox.ac.uk/orau/01_06.htm
There are several methods to radio carbon dating. They include the gas counting method of the 1950's, liquid scintillation counting, and accelerator mass spectrometry.
Liquid Scintillation Counting (LSC)
In the 1940's, it was found that certain organic compounds emit light when exposed to radiation. A fluorescence event is proportional to the decay event of C14; and the frequency is proportional to the number of C14 atoms in the sample.
The major scintillation solvent is benzene (C6H6) or benzene and toluene (C6H6CH3). (3) First the sample is converted to CO2 and reacted with molten lithium. This forms lithium carbide. (Li2C2).
2CO2 + 10Li -> Li2 + 4Li2O
The lithium carbide is heated to 800oC and placed under a vacuum for 30 minutes to remove un-reacted gasses and complete the synthesis. (3) Then it is cooled and acetylene gas is produced.
Li2C2 + 2H2O -> C2H2 + 2LiOH
Then using a catalyst such as silica-aluminum vandium activated catalyst benzene is formed. (3)
3C2H2 -> C6H6
The benzene is separated from the catalyst and stored in refrigeration to await counting. The benzene is transferred into counting vials that contain the sample solvent and scintillation. The most common scintillation is PPO + POPOP. The sample is transferred to a Quantulus spectrometer. There it is cooled and adapts to the dark for eight hours. Then the counting begins.
Accelerator Mass Spectrometry
Instead of measuring the radioactivity of the sample (as in LCS); Accelerator Mass Spectrometry (AMS) counts directly the radiocarbon atoms. The sample is converted into a beam of ions whose mass is measured by the addition of magnetic and electric fields. Because the concentration of C14 is so minute, it is difficult to use conventional mass spectrometry. An accelerator is used to remove ions that could be mistaken for C14. (4) This is a diagram of an accelerator.
http://www.rlaha.ox.ac.uk/orau/index.htm
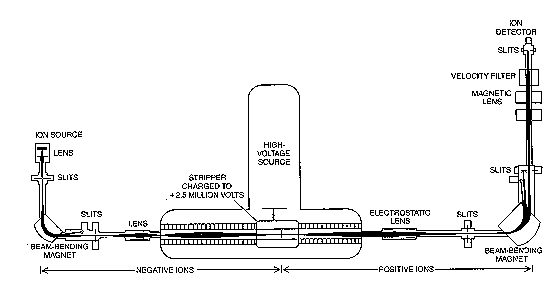
The sample is converted to graphite or carbon dioxide and then placed in the ion source. It is the ionized with cesium ions and focused into a beam. The first magnet selects ions with the mass of 14 (including 12CH2- and 13CH- ions). As the ions go through the accelerator, molecular ions are broken up. The second magnet selects ions with the expected momentum of 14C ions. (4) Then the number of 14C ions is counted.
Advantages and Disadvantages
With AMS the sample size needed for experimental determination of the age is typically 1000 times smaller. (4) One does not need to wait for the radiocarbon atoms to decay as they are counted directly. One problem with the small sample sizes is the increased risk of contamination. Therefore, calibration curves are based on conventional counting methods such as LSC because of the higher precision. If a large enough sample can be found LSC may be more precise. Today, however, there is a ‘minivial’ technique that allows radiocarbon dating of samples of less then one gram of carbon.
Testing a Sample
There are many labs that date samples. On average a single date costs about $250. An AMS machine costs about $2- $3 million dollars, plus the high fixed cost of upkeep.
Conclusion
There are several ways to date a sample and I hope I have given some insight to these processes. In conclusion, radiocarbon dating can be a very useful tool to scientists all around the globe. It allows people to see into the past and learn about ancient civilizations. Perhaps with proper use of this powerful tool we can learn about the past and look to the future.
Works Cited
Here are the references I used, including links to the websites.
(1) Jones, Lorreta and Peter Atkins. Chemistry: Molecules, Matter, and Change. (fourth edition.) New York: W.H. Freeman and Company, 1999: 579-583
(2) Thomas Higham. Radiocarbon Web Info/ Methods. (Online) www.c14dating.com/meths.html (11/10/02)
(3) Alan Hogg. Radiocarbon Web Info/ LSC (Online) www.c14dating.com/lsc.html (11/10/02)
(4) Oxford Radiocarbon Accelerator Unit (Online) www.rlaha.ox.ac.uk/orau.htm (11/10/02)