Summary of Current Research
1. Biphenyl
and Terphenyl “Smart” Biaryls: Dihedral Angle Modulation via pH and Redox
conditions
In this project we are synthesizing and studying bridged biphenyls and terphenyls. The goal is to understand if we can control dihedral angle reversibly and whether or not there is some distinguishable output depending on the dihedral angle of the biphenyl. By dihedral angle, we mean the angle between the two phenyl rings with the single bond as an axis. Fluorescence, color, and conductance are highly dependent on the dihedral angle of biphenyl compounds.
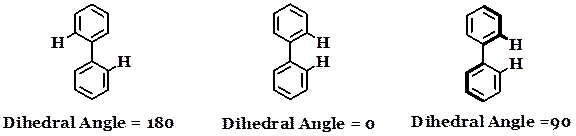
We are primarily interested in the synthesis and study of the fundamental properties of these compounds. However, these “Smart” Biaryls could be useful broadly as sensors, molecular electronic components, molecular machines and in other nanoscience applications. There are several possible “bridged” compounds in which the dihedral angle could be modulated.
This work has been
supported by the ACS-PRF and the Research Corporation for Scientific
Advancement. Currently it is supported by the UWEC Blugold Differential and the
Wisconsin Alliance for Minority Participation.
Publications:
Westlund, C. A.; Abdrabbo, A. G.;
Bruckbauer, A. M.; Gerlach, D. L.; Doyon, T. J.; Unruh, D. K.; Reinheimer, E.
W.; Dahl, B. J. Amino-Containing Donor-Acceptor Biaryls with Lactone Bridging
Units as Three-State Halochromic Pi-Expanded Coumarins. Tetrahedron, 2024,
154, 133889. link
Dressler, Justin J.;
Charlesworth-Seiler, Eva M.; Dahl, Bart J.* “Synthesis of a Triethylene
Glycol-Capped Benzo[1,2-c:4,5-c']bis[2]benzopyran-5,12-dione: A Highly Soluble
Dilactone-Bridged p-Terphenyl with a Crankshaft Architecture.” Tetrahedron
Lett. 2020, 61, 152429. link
Dressler, Justin J.;
Miller, Sarah, A.; Meeuwsen, Brian T.; Riel, Asia Marie S.; Dahl, Bart J.*
“Synthesis of Dilactone Bridged Terphenyls with Crankshaft Architectures.” Tetrahedron 2015, 71, 283-292. link
Carlson, Erik J.; Riel,
Asia Marie S.; Dahl, Bart J.* “Donor-Acceptor Biaryl Lactones: pH Induced
Molecular Switches with Intramolecular Charge Transfer Modulation.” Tetrahedron
Lett. 2012, 53, 6245-6249.
link
2. Soluble
Ladder and Star-Type Oligophenylenes
Oligophenyl compounds are excellent candidates for organic-type molecular wires and semiconductors. However, they do have many issues that need to be addressed, including solubility for processing purposes and poor conductance due to dihedral angles. Increasing the number of phenyl groups decreases solubility, and after n=3, oligophenyl molecules become nearly insoluble in all organic solvents. Additionally, because their dihedral angles are > 0, their conductance actually falls off quite drastically. In this project we hope to develop a synthesis of bridged oligophenyls which will hold their dihedral angles at 0 degrees and thus increase their conductance. Also, we propose to add solubilizing groups (long alkyl chains) that will aid in their solubility so that these compounds could be processed into useful materials.
The goal is to develop a modular and step-wise synthesis of bridged oligophenyls than have both linear and dendritic shapes. We will characterize them by UV-vis and fluorescence spectroscopy. Ultimately electrochemical studies will be done.
This work has been
supported by the ACS-PRF, Research Corporation for Scientific Advancement, the
UWEC Blugold Differential, and the Wisconsin Alliance for Minority
Participation.
Publications:
Hintz, Heather A.; Sortedahl, Nicholas J.; Meyer, Samantha M.; Decato,
Daniel A.; Dahl, Bart J.* “The Synthesis of
Lactone-Bridged 1,3,5-Triphenylbenzene Derivatives as Pi-Expanded Coumarin
Triskelions.” Tetrahedron Lett. 2017, 58, 4703-4708. link
3. Synthetic
Anthocyanidin-Type Molecules: Benzopyrylium-containing conjugated compounds
Anthocyanidins are common plant pigments based on the flavylium ion or 2-phenylchromenylium, which is a type of oxonium ion (chromenylium is referred also to as benzopyrylium). They are what give many fruits and flowers their colors. Natural anthocyanidins have recently been explored as components of dye-sensitized solar cells. These types of solar cells would be much cheaper, flexible, and possibly more efficient than current silicon-based solar cells. We would like to explore synthetic anthocyanidins with the same goal in mind.
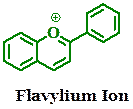
The goal is to develop a modular and step-wise synthesis of new benzopyrylium-containing conjugated compounds (synthetic anthocyanidins) and characterize them by UV-vis and fluorescence spectroscopy. The benzopyrylium moiety has been underutilized as an acceptor group. The pyrylium ion has well known pH-sensitivity as well and these compounds could be useful as sensors as well.
This work has been supported by the NSF-REU, ACS-PRF,
the Research Corporation for Scientific Advancement, the UWEC Blugold
Differential, and the Wisconsin Alliance for Minority Participation.
Publications:
Meyer,
Samantha M.; Charlesworth-Seiler Eva M.; Patrol, Joel G.; Kitzrow, Jonathan,
P.; Gerlach, Deidra A.; Reinheimer, Eric W.; Dahl, Bart J.* “Synthesis and Optical Properties of a Library of Isomeric
Aryldibenzopyrylium Halochromic Cations.” Tetrahedron
2020, 76, 131222. link
Prust, Erin E.; Carlson,
Erik J.; Dahl, Bart J.* “6-Aryldibenzo[b,d]pyrylium Salts: Synthesis and
Characterization of a Reversible pH-Driven Optical and Spectroscopic Response.”
Tetrahedron Lett. 2012, 53,
6433-6435. link
4. Synthesis of Protected Deuterated-Amino Acids to Study
Phosphorylation of Peptides by Nanoparticle-based SERS.
This was a
collaborative project with Dr. Laurie Parker
while at Purdue University and was funded through an NIH sub-contract. We were
working on developing a multistep synthesis of several protected deuterated
amino acids for Dr. Parker. The long-term
goal of this study is to
develop a sensitive, multiplexed detection platform for real-time single-cell
monitoring of prognostic kinase activity in tumor samples. The main objective
of this work was to develop the
first steps towards a multiplex quantification of kinase activity in a breast
cancer model system using SERS and peptide-functionalized nanoparticle
(NP)-based biosensors.
Last
updated: October 17, 2020