ADP-Ribosylation Factor, ARF, is a member of a superfamily of G-proteins the help in the binding of guanine nucleotides. The ARF family can be divided into at least six proteins which divide into three classes based on amino acid sequence, protein size, phylogenetic analysis, and gene structure. ARFs can reversibly associated with cytosolic surfaces of phospholipid membranes and have a covalently attached myristic acid group bonded at the N-terminus. ARF 1, which is the focus of my paper, is a surface protein and it binds to other proteins by insertion of a N-terminal myrisoyl chain. Myristoylated proteins have a 14-carbon chain saturated fatty acid a the N-terminal and are mostly associated with membranes. ARF proteins have no specificity as to what kind of lipids they bind too.
ARF interacts with membranes in two different ways: the myristate which binds with the protein membrane regardless of the conformation(either the GDP or the GTP conformation); and a protein region that becomes accessible upon binding of GTP which induces a conformational switch. Like all G-binding proteins, ARF 1 can have two different conformations according to whether or not GDP or GTP is bound to it. The N-terminal myristoylated end is exposed only after the GTP-bound conformation has changed. This conformation switch is the signal for G-proteins to bind to and activate their specific protein effector targets. It is not yet known how the GDP-GTP switch is made, how the myristoylated chain is bound to the ARF protein, or how the N-terminal is inserted into membrane.
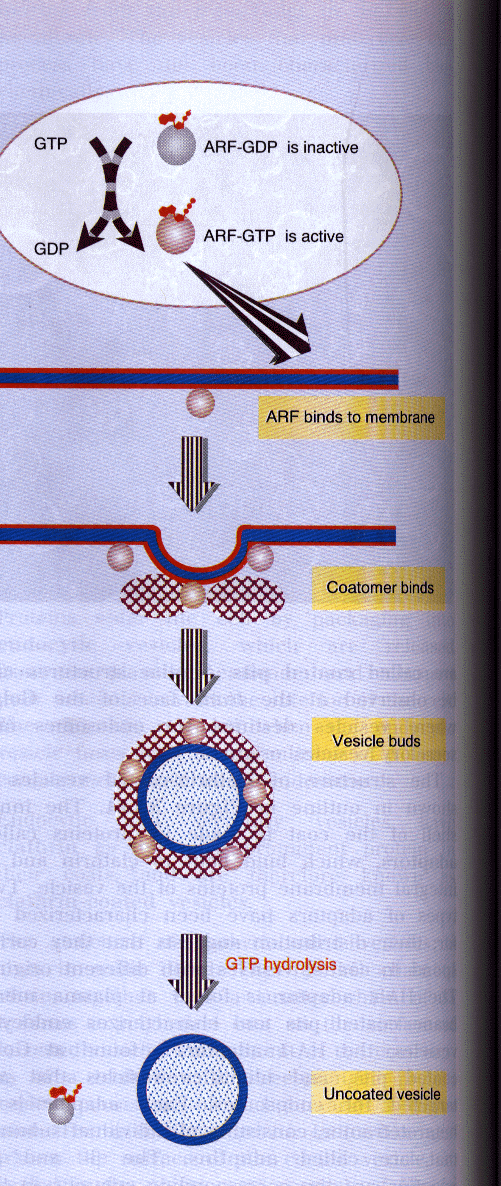
ADP-Ribosylation Factors play important roles in vesicular transport(see Figure at right)(Lewin, Genes 1038), maintenance and function of the Golgi structure, vesicular budding, and protein secretion. ARF is also involved in the Golgi derived formation of the non-clatherin-coated vesicles and the budding of clatherin and COP (coat protein)-I-coated vesicles. It is known that ARFs act as an activator for cholera toxin and phospholipase D(Zhang, etal., 1995). ARFs also make a large number of high-affinity interactions with ligands such as Mg+2 and phospholipids. It also interacts with other proteins like Gs and ARF-GAP. To view ARF1 in Van der Waals radius specify form click on button.