Triose Phosphate Isomerase
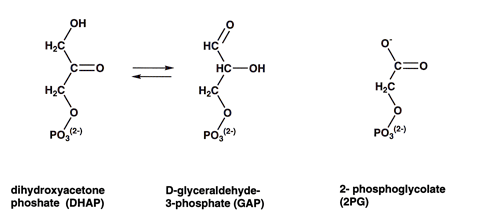
Triose phosphate isomerase (TIM) was selected to represent intramolecular oxidoreductases that catalyze the oxidation of one part of a molecule by the reduction of another part. In glycolysis dihydroxyacetone phosphate (DHAP) and D-glyceraldehyde-3-phosphate (GAP) are readily interconverted for glycolitic purposes. 2-phospohglycolate is a substrate analog which is used to show the active site.
TIM has been found in every organism that it has been look for in. Deficiency in triose phophate isomerase leads to high levels of dihydroxyacetone which can cause severe anemia and neourmuscular impairment. For those reason TIM has been extensively studied as a target in drug design. Specifically designed to fight against parasites of the human bloodstream like malaria.
TIM is a dimeric enzyme that does not require cofactors or metal ions for its activity. It has a globular shape formed by beta-strand, loop, alpha-helix, loop motif that is reproduced 8 times. The eight beta strands fold in way that produces a beta barrel with alpha-helices on the outside. This folding topology is found in many other enzymes but has adopted the name TIM barrel.
The TIM barrel enzymes usually have the active site at the C-terminal end of the beta-barrel and triose phosphate isomerase is no exception.
The eighth loop is the phosphate binding loop
because it contains a 3/10 helix (phosphate binding helix) whose N-terminus hydrogen bonds to the phosphate portion of either DHAP or GAP holding it in place so that the conversion at the triose end of the molecules can take place. The glutamate
on loop 6 is responsible for the transfer of a proton from one substrate carbon to another.