FAD or flavin adenine dinucleotide is a very common coenzyme (a cofactor made up of organic molecules) in proteins. Similar to NAD and NADP in that it carries electrons, FAD participates in many important chemical reactions that flavoproteins carry out.
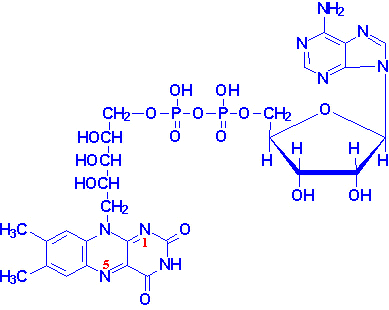
It is made up of the flavin backbone, two phosphate groups, an adenine, and a ribose. The molecular formula for FAD is C27H33N9O15P2. Because FAD can accept/donate one or two electrons it can participate in a wide range of oxidation-reduction reactions, as well as lyase and transferase reactions (to a much smaller degree).
The catalytic site of FAD is the isoalloxazine ring derived from riboflavin. The ribityl chain and ADP serve to stabilize FAD in enzymes, as FAD is usually bound to the enzymes at all times. This helps lower the energy of activation of many reactions, because FAD is already present in the protein and the reaction only depends on the enzyme finding the substrate. Here is the structure of FAD:
1. oxidized: has no hydrogens at positions N1 and N5 (FAD)
2. reduced: has hydrogens at both positions N1 and N2 (FADH2)
3. intermediate semiquinone: has one hydride ion at one of the positions (FADH- radical)
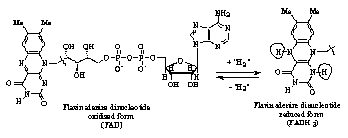
FAD can then be regenerated to its original form in the protein by external redox molecules.
See the role of FAD in these proteins :