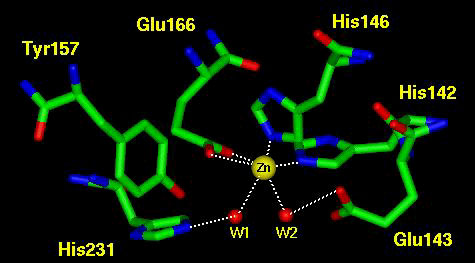
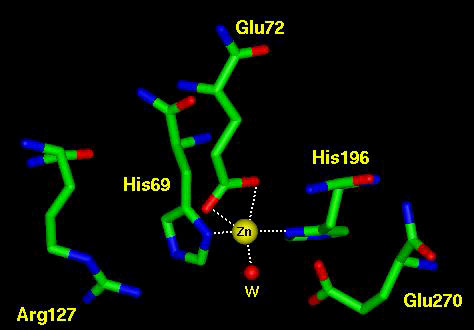
| Zinc is the second most abundant transition metal in biology, second only to iron. So far it has been found in over 300 different enzymes is many different species. It is essential to life and can be dietarily intaken from meats, eggs, spinach, onions, and oysters. It is very versatile as a cofactor, as its abundance implies. Zinc serves three general functions in biological systems. It can be structural, catalytic, or cocatalytic. It is structural as seen in the Zinc Finger motif commonly found in transcription factors. It is catalytic by itself in many enzymes, and it is also cocatalytic because it can perform an enzymatic catalysis function with another cofactor or metal.
Zinc is also important in biological structures because of its polarizable character, strong binding and lack of redox chemistry. The polarizable character can be seen in acitve sites such as B-lactamase and carboxypeptidase. The Zinc polarizes either water or a carbonyl bond for futher reaction. The lack of redox chemistry is important because it limits the "side reactions" of an enzyme. One notable "side reaction" is in hemoglobin, which is supposed to only carry oxygen to tissues. Occasionally, the oxygen oxidizes the iron to iron III which releases the dangerous superoxide radical into the blood, or cellular matrix. This kind of alternate reaction is much less likely with Zinc.
Enzymes with zinc active sites are inhibited by metal chelators such as EDTA, and can actually be replaced by some heavy metals like Lead and Mercury. In fact, this is thought to be the basis for lead and mercury poisoning. The Zinc can be functionally replaced by Cobalt II in some instances.
|
|