
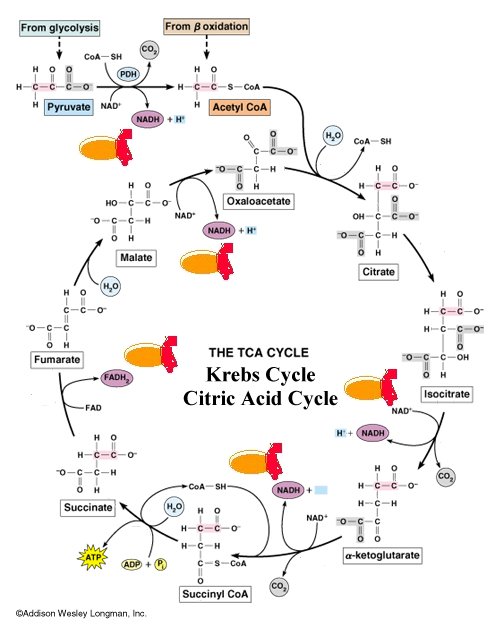
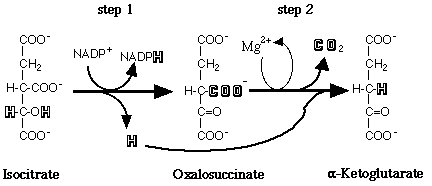
Isocitrate dehydrogenase (IDH) is an oxidoreductase that catalyzes an oxidation reduction in which isocitrate is converted to alpha-ketoglutarate and CO2. It is the first of four oxidative steps within the TCA cycle and is the key rate-limiting step of the TCA cycle. This conversion occurs in two steps: first, an oxidation of a secondary alcohol (isocitrate) to a ketone (oxalosuccinate), second, a beta-decarboxylation to produce alpha-ketoglutarate. (see below). Isocitrate dehydrogenase can be switched off by phosphorylation when the cell doesn't require energy production through the TCA cycle. Phosphorylation occurs directly at the active site on residue Ser 113.
IDH requires Mg2+, Mn2+; it is activated by ADP, Citrate, and Ca2+, and inhibited by NADH,NADPH, and ATP.
There are two forms of IDH that differ by coenzyme and localization within the cell. The IDH examined here is the predominant species with the NADP hetero group. It is found in all tissues of higher plants and animals and is thought to be the only form found in bacteria. it functions both in the cytosol and in the mitochondrial matrix. The other form utilizes NAD as its cofactor and is found only in the mitochondrial matrix. The mechanism of the NADP dependent form is illustrated on the "Tour of IDH".
 |