
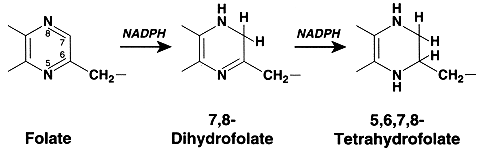
Dihydrofolate reductase (DHFR) catalyzes the NADPH linked reaction of 7,8-dihydrofolate to 5,6,7,8-Tetrahydrofolate. It is a ubiquitously expressed housekeeping enzyme and it is of great clinical importance. DHFR is principally responsible for maintaining cellular levels of tetrahydrofolate which is the cofactor used in synthesis of thymidylate, a building block of DNA. The requirement of folic acid for the growth of mammals, and especially for the development of blood cells, stimulated the pharmaceutical industry to undertake the preparation of analogues that might have anti-growth activity. Rapidly proliferating cells such as those in cancer require a large supply of deoxythymidylate . Inhibition of DHFR is the mechanism by which the antifolate drugs methotrexate (MTX) and aminopterin function. They are both potent competitive inhibitors of DHFR. Both are used for the treatment of a wide range of diseases including many cancers, infections, rheumatoid arthritis, multiple sclerosis and lupus. The success of methotrexate as a drug is due in large part to its strong binding to DHFR. Go to "Tour of DHFR"
 |
(PDB ID 1RB2)