|
Introduction/Reaction
|
|||||||||||||
 |
|||||||||||||
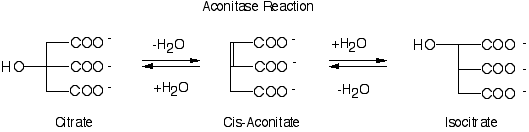
| Under aerobic conditions, many organisms convert the pyruvate obtained from glycolysis into acetyl-coenzyme A which is oxidized to CO2 in the citric acid cycle. The electrons made in the citric acid cycle are then passed through the electron transport chain to produce ATP.1 Aconitase, a dehydratase (EC 4.2.1.3), catalyzes the second step of the citric acid cycle (see above reaction) in which citrate is dehydrated to form the intermediate cis-aconitate and then rehydrated to form (2R,3S) isocitrate.8 Aconitase can also catalyze the reverse of this reaction converting isocitrate to citrate. The overall purpose of this reaction is to convert a tertiary alcohol (citrate) into a secondary alcohol (isocitrate) which is more easily oxidized to continue the citric acid cycle.1 Release of the product due to conformational changes of the protein is the rate-determining step of the reaction.10
Aconitase requires an iron-sulfur cluster cofactor which binds Fe2+ to activate the enzyme. The iron in this position binds to the C-3 carboxyl and hydroxyl groups of the substrate citrate and acts as a Lewis acid to make the hydroxyl a better leaving group. The reaction is reversible under physiological conditions, and the enzyme actually favors the reverse of the reaction that occurs in the citric acid cycle. The delta G0/ for the reaction is + 6.7 kJ/mol, and the delta G is +0.8 kJ/mol. Under equilibrium conditions, a mixture of substrate, transition state, and product would contain about 90% citrate, 4% cis-aconitate, and 6% isocitrate.1 Two diseases, myopathy and Wilson's disease, can be connected to low levels of aconitase activity. Myopathy is a disease characterized by abnormally low physical performance. Physical activity causes symptoms ranging from cramps, muscle fatigue, dyspnea, and palpitations to severe acidosis and myoglobinuria. Some forms of this disease can be linked to low levels of succinate dehydrogenase and aconitase, both of which contain iron-sulfur clusters. Decreased activity of these enzymes leads to a low rate of NADH production in the citric acid cycle and impaired muscle oxidative phosphorylation.3 Wilson's disease is characterized by a build up of copper in the liver and brain which causes hepatic and neurological abnormalities.4 Copper accumulation in the mitochondria of patients with Wilson's disease leads to a 71% decrease in aconitase activity which causes severe mitochondrial dysfunction in the liver.5 |
|||||||||||||
|
Introduction/Reaction
|
|||||||||||||