Electron Transfer and the Involvement of FAD and NAD+ in Lipoamide Dehydrogenase
Before we view the residues that are important in the oxidation of lipoamide, it is important to look at the molecules that receive and transfer the electrons. The coenzymes, FAD and NAD+ are the main participants in managing the flow of electrons from the amino acid residues that are the first recipients. Spectroscopic and kinetic experiments [6,7,10] were used to elucidate lipoadide dehydrogenase’s catalytic mechanism. As shown in figure 1, the ping-pong mechanism of lipoamide dehydrogenase can be broken down into 2 half-reactions; these reactions are completely reversible.
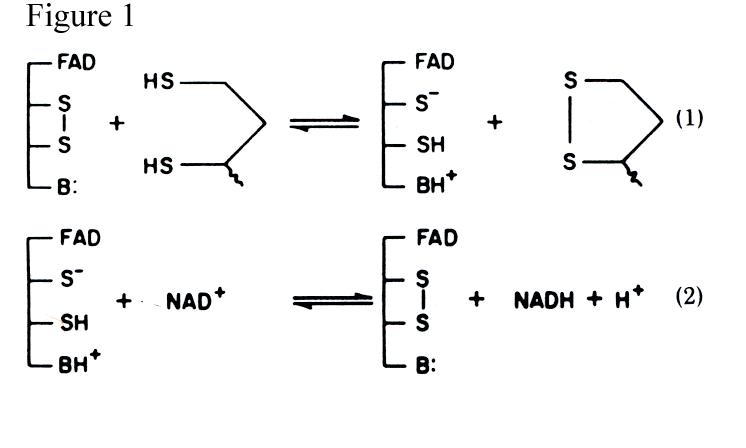
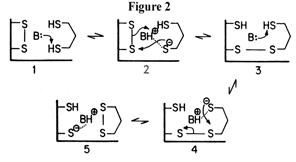
In the first reaction, dihydrolipoamide is oxidized by the enzyme, effectively reducing and opening the disulphide bridge between S48 and S53 with two electrons. Many biochemists have proposed mechanisms - one possible scheme given in figure 2, for the transfer of the electrons between these two groups.[10] Although mechanisms differ slightly, they all propose the participation of a base that activates the dihydrolipoamide by abstracting a proton from the reduced state. In reaction two, electrons are passed from the cysteine residues to the si-face of the flavin ring where they are accepted by NAD+ on the opposite re-face.[5] Thus, in the first half- reaction, the enzyme (E) is reduced by two electrons (EH2), and in the second half-reaction the enzyme passes these electrons to NAD+ to produce NADH and E.
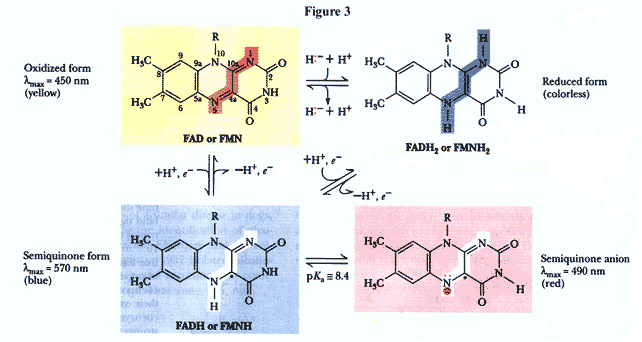
The entire structure of both FAD and NAD+ are needed for binding and function of the coenzymes; however, only the isoalloxazine and the nicotinamide rings, respectively, are directly involved in the passing of electrons. FAD’s isoalloxazine ring is especially important for the fact that it can transfer one or two electrons at a time. It can assume three different oxidation states because of the possible delocalization across the pi-electron system of the isoalloxazine ring (figure 3).
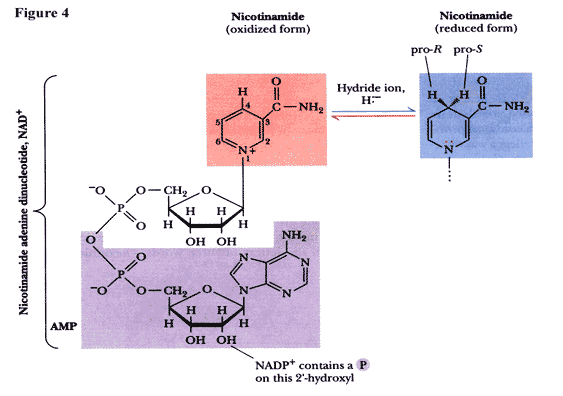
The forth carbon on the nicotinamide accepts two-electrons only in the form of a hydride anion (fig 4). Such versatility enables FAD to accept one or two electrons at a time from the enzyme where it can donate two electrons to NAD+ on the opposite side of the ring.[2]